A Novel Long Non-Coding RNA Regulates Tau Degradation
Neurodegenerative diseases such as frontotemporal dementia with tau inclusions (FTLD-tau), progressive supranuclear palsy (PSP), Alzheimer’s disease (AD), and Parkinson’s disease (PD) share a defining feature: their progression is driven by misfolded proteins that aggregate into toxic structures. In tauopathies, abnormal tau proteins accumulate into neurofibrillary tangles, while in synucleinopathies, alpha synuclein aggregates into insoluble Lewy bodies.
Despite decades of research into the molecular pathways underlying these conditions, the key upstream regulators of protein clearance remain unknown. To explore one potential regulator, scientists at Washington University in St. Louis, the University of California San Francisco, and the Chan Zuckerberg Imaging Institute examined the role of long non-coding RNAs (lncRNAs). Once dismissed as “junk DNA,” these non-protein-coding regions, representing more than 98% of the genome, have been shown through next-generation sequencing to play essential roles in biological regulation. Impressively, lncRNAs can influence gene expression, serve as molecular scaffolds or guides, and modulate post-transcriptional processes.
In a recent publication, Renganathan et al. investigated a novel lncRNA, FAM151B-DT, and shed light on its role in the clearance of toxic proteins implicated in neurodegeneration.
Monitoring lncRNA levels
In a previous study, the group identified the lncRNA FAM151B-DT and found that its expression is altered by mutations in the MAPT gene, which encodes tau. Specifically, the MAPT mutations IVS10+16, P301L, and R406W led to reduced FAM151B-DT expression in iPSC-derived neurons compared with isogenic controls. Post-mortem analyses mirrored these findings in human disease. FAM151B-DT levels were significantly lower in brain tissue from patients with FTLD-tau, PSP, and AD than in healthy individuals.
By demonstrating consistent downregulation of FAM151B-DT across both in vitro and in vivo models, the authors identified FAM151B-DT as a shared, early marker of tau pathology.
Regulation of tau seeding
To determine whether FAM151B-DT influences tau’s seeding capacity, a tau biosensor cell line expressing a P301S-mutated tau fragment was tagged with either CFP or YFP. When tau aggregates came into proximity with the CFP- and YFP-tagged fragments, a FRET signal was produced upon excitation. FAM151B-DT knockdown was achieved by transfecting cells with siRNA. To assess the impact on tau seeding, cells were then treated with StressMarq’s Tau-441 (2N4R) Wild-Type Pre-formed Fibrils (catalog# SPR-480), which act as a template to convert soluble, monomeric tau into misfolded, aggregated forms.
Renganathan et al. found that reducing FAM151B-DT expression by ~50% significantly increased tau seeding and aggregation, as measured by FRET signal intensity relative to controls. Conversely, in an overexpression model where FAM151B-DT was elevated instead of silenced, treatment with tau pre-formed fibrils produced the opposite outcome, with a four-fold increase in FAM151B-DT expression reducing tau seeding by half.
These functional assays demonstrate that FAM151B-DT directly regulates tau seeding. The knockdown phenotype mirrors conditions in MAPT-mutant brains, where reduced FAM151B-DT expression coincides with heightened tau aggregation.
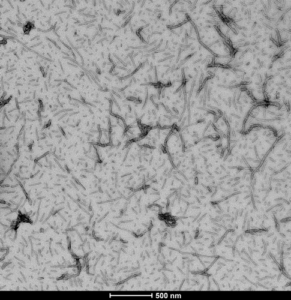
Figure 1. [Image from: StressMarq website] Transmission electron microscopy (TEM) imaging of Tau-441 (2N4R) Wild-Type Pre-formed Fibrils (catalog# SPR-480).
Formation of a functional complex
The scientists next conducted a detailed characterization of FAM151B-DT. Evolutionary analyses revealed that while FAM151B-DT is poorly conserved across vertebrates, it is detectable in most human tissues. Brain specificity data showed its highest expression in neurons, though it is also present in glia, microglia, oligodendrocytes, and endothelial cells, indicating broad expression throughout the central nervous system. The lncRNA did not exhibit any cis-regulatory effects in iPSC-derived neurons or SH-SY5Y cells.
Subcellular fractionation in both models demonstrated that FAM151B-DT localizes predominantly to the cytoplasm, suggesting a role in post-transcriptional regulation or in scaffolding protein complexes. To test whether it could function as a scaffold for tau, immunoprecipitation assays were performed. The resulting data confirmed the presence of a physical interaction between FAM151B-DT and tau.
To identify additional binding partners, the authors utilized Comprehensive Identification of RNA-Binding Proteins by Mass Spectrometry (ChIRP-MS), uncovering 121 high-confidence FAM151B-DT interactors in SH-SY5Y cells. Further analysis revealed that many of these proteins participate in cellular homeostasis and are directly connected to tau binding, autophagy pathways, and vesicle dynamics – core processes disrupted in neurodegenerative disease.
Futhermore, pull-down assays and ChIRP-MS mapping also showed that FAM151B-DT interacts with the chaperone HSC70, which promotes protein folding, stabilization, and lysosomal degradation. Structural mapping of the interaction sites suggested that FAM151B-DT may serve as a scaffold that brings tau and HSC70 into proximity, supporting tau’s delivery to degradation pathways.
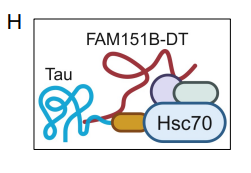
Figure 2. Schematic representation of interaction between FAM151B-DTl, tau, and HSC70. (Figure taken from Renganathan, A. et al., 2025 used under license CC BY 4.0).
Effect on lysosomal and autophagic pathways
Renganathan et al. next examined how alteration of FAM151B-DT levels affects autophagy in SH-SY5Y cells. When FAM151B-DT was silenced, HSC70 and LAMP1 protein levels remained unchanged. In contrast, LAMP2A levels and the LC3-II/I ratio increased. An elevated LC3-II/I ratio typically reflects enhanced autophagy initiation; however, accumulation of p62, which is degraded during autophagy, also increased. Levels of phosphorylated tau (p-tau) and total tau rose as well, indicating impaired autophagosome-lysosome fusion and inefficient protein degradation.
This accumulation of p-tau and p62 parallels pathological signatures observed in patient brains and suggests a mechanistic link between reduced FAM151B-DT and enhanced tau seeding.
Conversely, overexpression of FAM151B-DT increased lysosomal markers LAMP1, LAMP2A, and HSC70, while reducing p62 and the LC3-II/I ratio, consistent with restored autophagic flux. Notably, FAM151B-DT overexpression also lowered p-tau levels without altering total tau, demonstrating that elevated FAM151B-DT promotes the autophagic degradation of misfolded tau.
Interaction with alpha synuclein
To explore whether FAM151B-DT might also regulate other neurodegeneration-associated proteins, the researchers performed in silico motif screening. It was determined that more than 80% of FAM151B-DT-interacting proteins contain KFERQ-like motifs, which are recognized by HSC70 to target substrates for chaperone-mediated autophagy. This suggests that FAM151B-DT may cooperate with HSC70 to deliver a broad range of proteins for autophagic clearance.
Among the proteins containing KFERQ-like motifs is alpha synuclein, a crucial protein that aggregates to form Lewy bodies in Parkinson’s disease. Using ChIRP-MS and RNA immunoprecipitation, the authors confirmed that FAM151B-DT directly binds alpha synuclein, implicating this lncRNA in synucleinopathy pathways. Patient brain analyses further showed reduced FAM151B-DT expression in cortical samples from individuals with Parkinson’s disease.
Functional assays further supported these observations, allowing researchers to conclude that silencing FAM151B-DT results in alpha synuclein accumulation, whereas overexpressing FAM151B-DT promotes the clearance of the phosphorylated protein.
Summary
In summary, Renganathan et al. have identifed FAM151B-DT as a key regulator of autophagic clearance that links tau and alpha synuclein pathology through its interactions with the HSC70 chaperone system. The findings of their study posit that FAM151B-DT levels are reduced in patient brain tissue and in stem-cell models of tauopathy. Notably, restoring FAM151B-DT expression reinstates autophagic degradation of both tau and alpha synucleuin. By shifting FAM151B-DT levels, cells effectively toggle between impaired and functional autophagy, determining whether toxic proteins accumulate or are cleared. These findings open new therapeutic avenues aimed at enhancing FAM151B-DT expression to boost autophagy across multiple neurodegenerative disease pathologies.
Related StressMarq products
Continued research into tauopathies is vital for the advancement of early disease diagnosis and drug discovery. To support this research, StressMarq Biosciences manufactures an extensive selection of high-quality reagents, including monomeric, oligomeric, and fibrillar tau protein constructs. The hyperphosphorylated Tau-441 (2N4R) Wild-Type Pre-formed Fibrils (Baculovirus/Sf9) (catalog# SPR-498) and Tau-441 (2N4R) Wild-Type Oligomers (Baculovirus/Sf9) (catalog# SPR-497) are ideal for seeding assays where a toxic tau species is required. Visit our website for more information, including the latest scientific publications using our specialized tau, amyloid beta and alpha synuclein proteins.
References
- A novel lncRNA FAM151B-DT regulates degradation of aggregation prone proteins. Renganathan, A. et al., Mol Psychiatry. 2025.
- A novel lncRNA FAM151B-DT regulates autophagy and degradation of aggregation prone proteins. Renganathan, A. et al., medRxiv [Preprint]. 2025


Leave a Reply