Microglial Receptors Mediate Tau Clearance
Tau is the predominant microtubule-associated protein (MAP) in mature, healthy neurons, where it plays a critical role in stabilizing microtubules and supporting neuronal structure and function. In Alzheimer’s disease (AD) and other tauopathies, tau undergoes abnormal post-translational modifications and conformational changes, resulting in the formation of pathological aggregates such as tau oligomers and fibrils. These aggregates, commonly referred to as neurofibrillary tangles (NFTs), do not distribute uniformly across the brain. Instead, they initially accumulate in the entorhinal cortex, a region essential for memory processing, and subsequently spread to the hippocampus and neocortex. This progression closely correlates with clinical symptoms, including cognitive decline and memory impairment.
The propagation of tau pathology is facilitated by neuron-to-neuron transmission via secretory mechanisms, allowing misfolded tau species to seed the aggregation of soluble endogenous tau into insoluble fibrillar forms. This prion-like spread is a key driver of the progressive nature of tau-related neurodegeneration. Emerging evidence highlights the pivotal role of microglia, the resident immune cells of the central nervous system, in modulating tau pathology. Microglia can limit tau propagation by internalizing and degrading extracellular tau aggregates through mechanisms such as phagocytosis.
Several innate immune receptors, including complement receptor 3 (CR3) and complement receptor 4 (CR4), have been implicated in the clearance of neurotoxic proteins. Notably, a study by Yoo et al. demonstrated that CR4 selectively binds pathological tau fibrils, but not physiological tau monomers, facilitating their uptake and degradation via phagocytosis. These findings suggest that CR4-mediated clearance of tau aggregates may represent a promising therapeutic target for Alzheimer’s disease and other tauopathies.
CR4-tau interaction
The research team, comprising investigators from the Korea Brain Research Institute (KBRI), Daegu Gyeongbuk Institute of Science and Technology, and Korea Basic Science Institute (KBSI), employed recombinant tau proteins in a preliminary dot blot assay to investigate the interaction between CR4 and distinct tau species. Specifically, various concentrations of StressMarq’s Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (catalog# SPR-329) and Tau-441 (2N4R) P301S Mutant Monomers (catalog# SPR-327) were blotted onto a nitrocellulose membrane and incubated with recombinant CR4. The fibrillar tau species contained the P301S missense mutation, which is associated with neurodegenerative tauopathies.
CR4 is a heterodimeric integrin composed of ITGAX (CD11c, α-chain) and ITGB2 (CD18, β-chain). To detect potential protein-protein interactions, antibodies targeting these subunits were employed. The assay revealed that CR4 selectively binds to tau fibrils in a dose-dependent manner, while showing no affinity for monomeric tau, indicating specificity for the pathological multimeric form. To further assess receptor specificity, CR3, another CD18 integrin with a similar heterodimeric structure and commonly co-expressed with CR4, was included in the analysis. Despite exposure to high concentrations of tau fibrils, CR3 failed to exhibit any binding, reinforcing the selective interaction between CR4 and tau fibrils.
The CR4-tau interaction was subsequently validated in a cellular context. HEK293 cells were engineered to express epitope-tagged CR4 heterodimers and incubated with tau fibrils. Confocal microscopy and immunoprecipitation confirmed the formation of a biologically relevant CR4-tau complex, providing further evidence of CR4’s role in recognizing and potentially mediating the clearance of pathological tau aggregates.
Reduction of tau uptake and clearance
Subsequently, the focus of the study shifted to examining the role of CR4 in the microglial clearance of extracellular tau via phagocytosis. To this end, Yoo et al. utilized the BV2 microglial cell line – a murine model known for its robust phagocytic activity and inflammatory responsiveness. This aspect of tau clearance is critical, as microglia can prevent the propagation of tau pathology between neurons. In the absence of such clearance, extracellular tau fibrils may be internalized by neurons, triggering the conversion of native monomeric tau into pathological aggregates.
To visualize the phagocytic process, tau fibrils were labeled with a pH-sensitive rhodamine-based dye. In the extracellular neutral environment, fluorescence remained low; however, upon internalization into acidic compartments such as mature phagosomes within microglia, fluorescence intensity increased, providing a dynamic readout of phagocytosis.
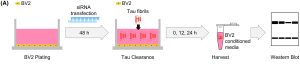
Figure 1. Schematic structure of the clearance assay of extracellular tau fibrils by BV2 cells. (Figure taken from Yoo, C. J., et al, 2024 used under license CC BY 4.0).
In order to assess the specific involvement of CR4, the expression of Itgax, the gene encoding the CD11c α-chain of the CR4 heterodimer, was silenced using siRNA. This knockdown led to a marked decrease in intracellular acidification, indicative of impaired phagosome maturation, which could be further inhibited by a known phagocytosis inhibitor.
Importantly, CR4 silencing selectively impaired microglial phagocytosis of tau fibrils without affecting other tau clearance mechanisms, such as extracellular degradation by proteases like matrix metalloproteinase 9 (MMP9). These findings provide compelling evidence that CR4-mediated internalization is a key mechanism by which microglia clear extracellular tau fibrils, thereby potentially limiting the spread of tau pathology in neurodegenerative diseases.
Increased seeding capacity
In order to conclude the study, it was crucial to determine whether modulation of CR4 could influence the clearance of extracellular tau and thereby affect the misfolding and aggregation of physiological tau. This was assessed using conditioned medium, containing extracellular tau fibrils and other secreted cellular components, collected from BV2 microglial cells with CR4 silencing.
The conditioned media were applied to a fluorescence resonance energy transfer (FRET)-based biosensor cell line engineered to express the tau repeat domain (RD) with the P301S mutation, fused to cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP).
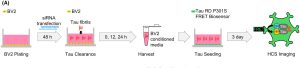
Figure 2. Schematic structure of tau seeding assay with BV2 conditioned media using tau RD FRET biosensor cells. (Figure taken from Yoo, C. J., et al, 2024 used under license CC BY 4.0).
If extracellular tau fibrils are internalized by these biosensor cells, they can seed aggregation of the tau RD P301S, bringing the CFP and YFP tags into close proximity. This process enables FRET, which can be quantitatively monitored. Experimental results demonstrated that CR4 silencing in microglia significantly increased the seeding capacity of extracellular tau, leading to enhanced tau aggregation in the biosensor cells.
Identifying microglial receptors with StressMarq’s neurodegenerative protein constructs
Microglial receptors are essential for the clearance of extracellular toxic molecules in the central nervous system. However, until recently, it remained unclear whether these receptors could also internalize pathological extracellular tau fibrils and mediate their degradation via phagocytosis. Using StressMarq’s Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (catalog# SPR-329) and Tau-441 (2N4R) P301S Mutant Monomers (catalog# SPR-327), researchers provided the first evidence that CR4, a complement receptor expressed on microglia, selectively binds to extracellular tau fibrils and facilitates their uptake and clearance through phagocytosis. This discovery highlights a previously unrecognized mechanism by which microglia may limit the spread of tau pathology and offers a promising therapeutic target for neurodegenerative diseases such as Alzheimer’s disease.
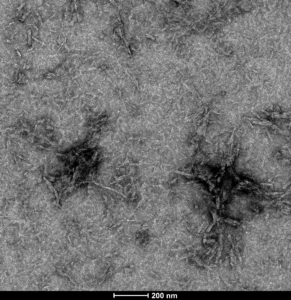
Figure 3. [Image from: StressMarq website] Transmission electron microscopy (TEM) imaging of Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (catalog# SPR-329).
Summary
Tau propagation and the progression of neurodegenerative diseases are closely linked to tau secretion. Extracellular tau fibrils facilitate cell-to-cell transmission, contributing to neurite degeneration and memory impairment. In their study, Yoo et al. identified a critical role for complement receptor 4 in selectively recognizing and binding pathological tau fibrils, but not monomeric tau. This interaction enables microglial clearance of extracellular tau fibrils via phagocytosis.
Importantly, inhibition of CR4 in microglia had detrimental effects, significantly impairing the clearance of extracellular tau fibrils. The study’s findings underscore the therapeutic potential of targeting CR4 to enhance microglia-mediated clearance of pathological tau. By promoting the removal of extracellular tau fibrils, CR4 may serve as a promising avenue for intervention in tauopathies such as Alzheimer’s disease and frontotemporal dementia.
Related StressMarq products
StressMarq Biosciences proudly offers a diverse selection of high-quality reagents to support neurodegenerative disease research, including monomeric, oligomeric, and fibrillar tau protein constructs. In addition, StressMarq’s unique selection of tau antibodies, such as the human tau 2N isoform-specific Tau 2N (2N3R/2N4R) Antibody (catalog# SPC-813), supports a wide range of research applications. Visit our website for more information, including the latest scientific publications using our specialized tau, amyloid beta and alpha synuclein proteins.
References
- Complement receptor 4 mediates the clearance of extracellular tau fibrils by microglia. Yoo, C. et al. FEBS Journal. 2024.


Leave a Reply