Structural Determinants of Tau Modulate Seeding Efficiency
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the global leading cause of dementia. Its pathology is characterized by the accumulation of misfolded proteins in the brain. The two key hallmarks of AD are the presence of extracellular amyloid-beta plaques and intracellular tau tangles, with tau aggregation being more closely associated with disease progression.
Tau, encoded by the MAPT gene, is a natively unfolded protein that contains three or four microtubule-binding repeats. It plays a crucial role in stabilizing microtubules, crucial components of the cellular cytoskeleton, and is regulated by post-translational modifications, particularly phosphorylation. Interestingly, when tau becomes hyperphosphorylated, it detaches from microtubules and assembles into the amyloid filaments commonly found in the brains of Alzheimer’s patients.
These hyperphosphorylated tau proteins misfold into beta-sheet-rich structures that ultimately aggregate into paired helical filaments. The multi-step process, known as seeding, allows the filaments to act as templates, inducing additional tau monomers to misfold and aggregate into obstructive neurofibrillary tangles.
Investigating tau structural variability with StressMarq’s neurodegenerative protein constructs
An exciting new study by Kasen et al. investigates whether the seeding capacity of misfolded tau filaments depends on a specific core structure, and whether post-translational modifications to the disordered outer “fuzzy coat” play a role. Conducted as part of an ongoing collaboration with the Van Andel Institute (Grand Rapids, USA), the study explores how tau structure influences its seeding potential. Using a tissue culture model of cortical neurons derived from healthy mice, researchers compared the seeding efficiency of recombinant tau proteins to that of tau extracted from an Alzheimer’s patient (AD tau).
The study also focused on two specific StressMarq proteins: RNA-induced Tau-441 (2N4R) P301S Mutant Filaments (catalog #SPR-463) & heparin-induced Tau-441 (2N4R) Wild-Type Pre-formed Fibrils (catalog #SPR-480). Among the samples tested, AD tau treatment led to the highest levels of tau accumulation. In contrast, the recombinant tau fibrils induced only minimal seeding. This discrepancy is hypothesized to stem from structural differences in the core regions of the recombinant filaments compared to those found in tau aggregates isolated from Alzheimer’s disease patients.
Elucidating differences in the tau core
Three additional recombinant tau filaments—4a, 42ab, and 12a—were evaluated, one of which (4a) was confirmed to share the ordered core structure characteristic of Alzheimer’s disease (AD) tau. It is well established that distinct tauopathies are associated with unique tau folding patterns. When the initial study was repeated, filament 4a exhibited greater seeding capacity than the other two recombinant filaments, although still significantly less than AD tau. This finding underscores the critical role of the ordered core structure in facilitating seeding activity.
Previous studies have demonstrated that injection of AD tau into healthy mice can induce aggregation of endogenous mouse tau. To assess the in vivo seeding potential of the recombinant filaments, the three variants were injected into healthy mice and evaluated nine months post-injection, alongside an AD tau control. Mice injected with 42ab and 12a showed no evidence of tau pathology, while those receiving 4a displayed only minimal tau accumulation.
Evaluation of seeding dynamics
To determine whether seeding efficiency was time-dependent, a second cohort of mice was assessed 18 months post-injection. In this group, tau accumulation induced by 4a increased, suggesting that 4a filaments seed more slowly than AD tau.
Finally, an evaluation of the influence of species-specific tau isoform expression on seeding capacity was performed using transgenic mice expressing the human MAPT gene. In these mice, both AD tau and 4a filaments exhibited enhanced seeding activity, whereas 42ab and 12a again failed to induce tau pathology. These results suggest that both the structural features of tau filaments and the host’s tau isoform expression modulate seeding efficiency.
Phosphorylation of the tau fuzzy coat
The disordered N- and C-terminal regions of tau, collectively referred to as the fuzzy coat, are the primary sites for post-translational modifications. Notably, the recombinant tau filament 4a lacks this fuzzy coat. To investigate whether its absence contributed to the reduced seeding capacity observed in earlier experiments, researchers treated AD tau filaments with either pronase (to remove the fuzzy coat) or phosphatase (to remove phosphorylation). Importantly, these treatments do not alter the filament’s ordered core structure.
Primary cortical neurons were seeded with untreated, phosphatase-treated, or pronase-cleaved AD tau. Both dephosphorylation and fuzzy coat removal resulted in more than a four-fold reduction in seeding capacity, underscoring the importance of these modifications in tau propagation.
To evaluate whether these modifications also affect in vivo seeding kinetics, MAPT transgenic mice were injected with treated or untreated AD tau and assessed nine months post-injection. Mice receiving treated AD tau exhibited reduced tau pathology compared to those injected with untreated AD tau, with pathology levels closely resembling those observed in mice injected with 4a filaments.
Modulation of phosphorylation events
To further explore the role of fuzzy coat phosphorylation in seeding, dephosphorylated AD tau filaments were re-treated with kinases to restore phosphorylation at sites commonly hyperphosphorylated in Alzheimer’s disease. When primary cortical neurons were seeded with untreated, dephosphorylated, or rephosphorylated AD tau, the rephosphorylated filaments showed a partial rescue of seeding capacity.
Additionally, a recombinant full-length tau variant containing 12 phosphomimetic mutations (PAD12 tau), which mimic phosphorylation events in disease pathology, was tested. PAD12 tau forms filaments with an ordered core identical to that of AD tau and retains a fuzzy coat. When seeded alongside AD tau in primary cortical neurons, PAD12 tau matched AD tau’s seeding efficiency at lower concentrations and outperformed all other recombinant tau filaments by at least tenfold.
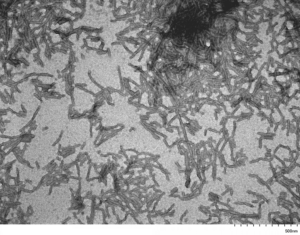
[Image from: StressMarq website] Transmission electron microscopy (TEM) imaging of Tau-441 (2N4R) P301S Mutant Filaments (catalog# SPR-463).
Summary
This study aimed to determine whether the filament core structure of tau influences its seeding capacity, and to assess the role of the surrounding disordered region—known as the fuzzy coat—in this process. The results demonstrate that the specific ordered core structure characteristic of Alzheimer’s disease tau filaments is essential for efficient seeding. However, the fuzzy coat also plays a critical role. In particular, post-translational modifications within this region, particularly phosphorylation, are necessary to support robust tau propagation.
These findings contribute to the broader understanding of neurodegenerative disease mechanisms by identifying structural and biochemical features of tau that are critical for its pathological spread. This insight may inform the development of targeted therapies aimed at disrupting tau propagation in tauopathies such as Alzheimer’s disease.
Related StressMarq Products
StressMarq manufactures a wide range of neurodegenerative proteins in different conformations, providing researchers with the high-quality tools needed to develop robust assays for neurodegenerative disease studies. Visit our website for more information, including the latest scientific publications using our specialized tau, amyloid beta and alpha synuclein proteins for neurodegenerative disease research. Of particular relevance are the Tau dGAE (297-391) AD-mimic Pre-formed Fibrils (catalog# SPR-502), fibrilized under highly-specific conditions in order to replicate the distinct structural fold found in AD tau.
References
- Seed structure and phosphorylation in the fuzzy coat impact tau seeding competency. Kasen, A. et al., bioRxiv [Preprint]. 2025.

Leave a Reply