Exposing the Links Between Lysosomal Membrane Repair and Age-Related Diseases
Long known for their role in the digestion of unwanted intra- and extracellular components, lysosomes are membrane-bound organelles now recognized for their additional roles as signaling centers, mediating nutrient sensing and calcium regulation. Stresses to cells can damage the lysosomal membrane causing the release of hydrolytic enzymes used for lysosomal digestion. This is harmful to cells and can induce cell death by necrosis. Therefore, cells must have pathways that promptly repair the lysosomal membrane.
Until now, the repair mechanisms used by the lysosomal membrane have remained poorly understood. In a recent study published in Nature, Tan et al. describe the pathway underlying the rapid repair of the lysosomal membrane. The authors also suggest a role of this repair pathway in age-related diseases with impaired lysosomal function, such as Alzheimer’s disease (AD)1.
Understanding the lysosomal repair mechanism
To understand how the lysosomes can be repaired, the researchers from the Nature study used a chemical agent that damaged the lysosomal membrane. The study used an unbiased proteomic approach that labelled proteins with biotin. Biotinylated proteins were purified and identified using mass spectrometry. By comparing which proteins were recruited to normal and damaged lysosomes, the researchers could identify proteins that play essential roles in the repair pathway.
The study methods proved effective, as they identified proteins including those well-known to be recruited to damaged lysosomes. Mass spectrometry then identified phosphatidylinositol-4 kinase type 2α (PI42K2A), lipid messenger proteins, and other pathway members. The researchers elucidated the pathway that rapidly repaired lysosomes using subcellular localization studies and protein analysis.
There are distinct stages in the pathway. First, PI42K2A rapidly accumulates on the damaged lysosome. This results in the generation of high levels of phosphatidylinositol-
4-phosphate (PtdIns4P) lipid messengers, which in turn recruit multiple oxysterol-binding proteins (OSBP)-related protein (ORP) family members. These ORP proteins set up contact sites between the endoplasmic reticulum and damaged lysosomes. The ORP proteins catalyze the ER-to-lysosome transfer of phosphatidylserine (PS) and cholesterol molecules. Finally, lysosomal PS accumulation recruits ATG2 in an autophagy-independent process. ATG2 serves to transport lipids for rapid lysosomal repair.
Physiological significance of lysosomal repair pathways
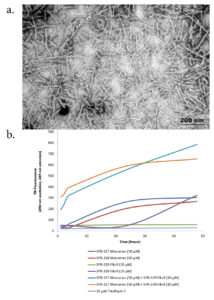
Figure 1. (a) TEM of recombinant Tau441 (2N4R) P301S mutant PFFs (catalog# SPR-329). (b) Thioflavin T emission curves show increased fluorescence (correlated to tau aggregation) when Tau441 (2N4R) P301S mutant PFFs (catalog# SPR-329) are combined with tau monomers (catalog# SPR-327).
The first protein in the lipid pathway recruited to damaged lysosomes is PI42K2A. PI42K2A produces a neurodegenerative phenotype in knockout mice, and mice show symptoms of tremors, limb weakness, urinary incontinence, and premature mortality2. StressMarq’s Tau-441 Mutant Pre-formed Fibrils (catalog# SPR-329) were employed to decipher whether PI42K2A in the lipid pathway also influenced age-related diseases.
In neurodegenerative diseases such as AD, tau aggregates can transfer or spread between cells to induce misfolding and aggregation of healthy tau molecules in previously healthy cells. StressMarq’s tau pre-formed fibrils induce the cell-to-cell spread of tau aggregates.
To visually monitor tau spreading, Tan et al., added sonicated tau pre-formed fibrils to cells stably expressing fluorescent tau monomers and control cells. Tau spreading was monitored by calculating the percentage of cells with fluorescence vesicles after tau fibril exposure. Depletion of PI42K2A showed an increase in the number of cells exhibiting tau spreading after exposure to fibril tau.
The research by Tan et al. may lead to new therapeutic targets for age-dependent diseases characterized by damaged lysosomes.
References
- A phosphoinositide signaling pathway mediates rapid lysosomal repair, Tan, J. & Finkel, T. Nature. 2022; 609(7928): 815–821.
- Loss of phosphatidylinositol 4-kinase 2α activity causes late onset degeneration of spinal cord axons, Simons, J. et al., Proceedings of the National Academy of Sciences of the United States of America. 2009; 106(28).


Leave a Reply