| Product Name | DNA Damage (8-OHdG) ELISA Kit |
| Description |
Colorimetric detection of 8-hydroxy-2-deoxy Guanosine |
| Species Reactivity | Species Independent |
| Platform | Microplate |
| Sample Types | Cell lysates, Plasma, Sample matrices, Urine |
| Detection Method | Colorimetric Assay |
| Assay Type | Competitive ELISA (Enzyme-linked Immunosorbent Assay) |
| Utility | ELISA Kit for 8-OHdG detection in samples. |
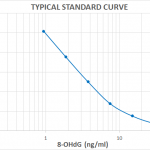
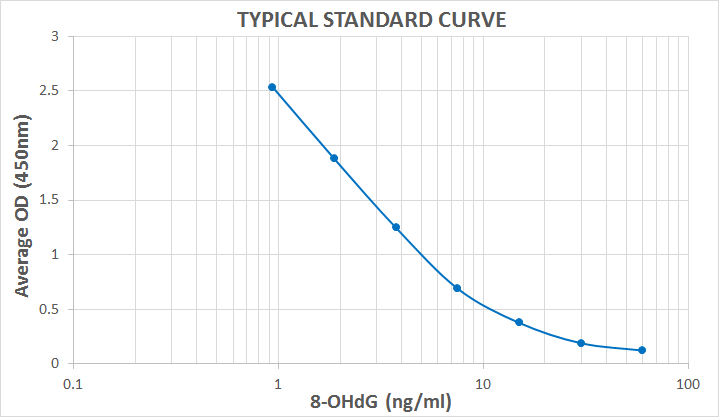
| Sensitivity | 0.59 ng/mL |
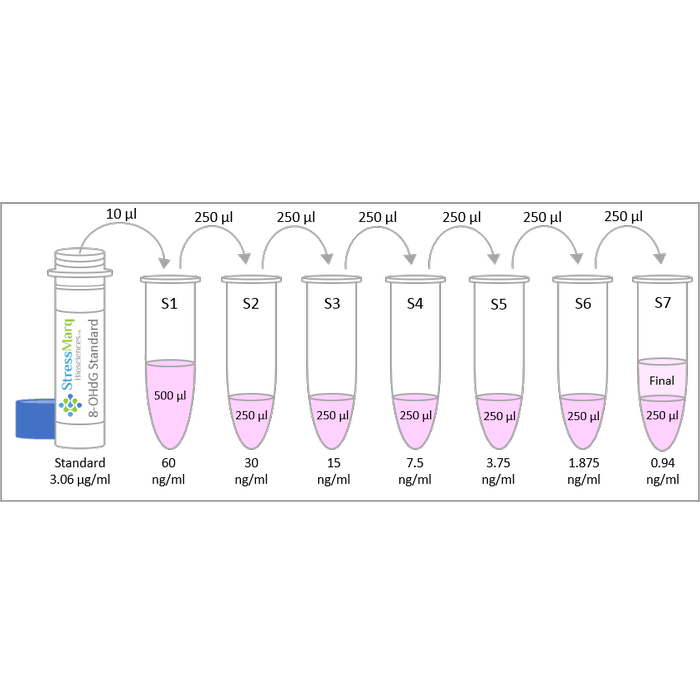
| Assay Range | 0.94 - 60 ng/mL |
| Precision | Intra-Assay Precision: Three samples of known concentration were assayed thirty times on one plate; the intra-assay coefficient of variation of the DNA Damage ELISA has been determined to be <5%. Inter-Assay Precision: Three samples of known concentration were assayed thirty times in three individual assays; the inter-assay coefficient of variation of the DNA Damage ELISA has been determined to be <5%. |
| Incubation Time | 1 hour |
| Number of Samples | 39 samples in duplicate |
| Other Resources | Kit Booklet - Storage Conditions Updated May 2024 , MSDS , DNA Damage ELISA Kit Calculations Worksheet |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | 4ºC and -20ºC | ||||||||||||||||||||||||||||||
| Shipping Temperature | Blue Ice | ||||||||||||||||||||||||||||||
| Product Type | ELISA Kits | ||||||||||||||||||||||||||||||
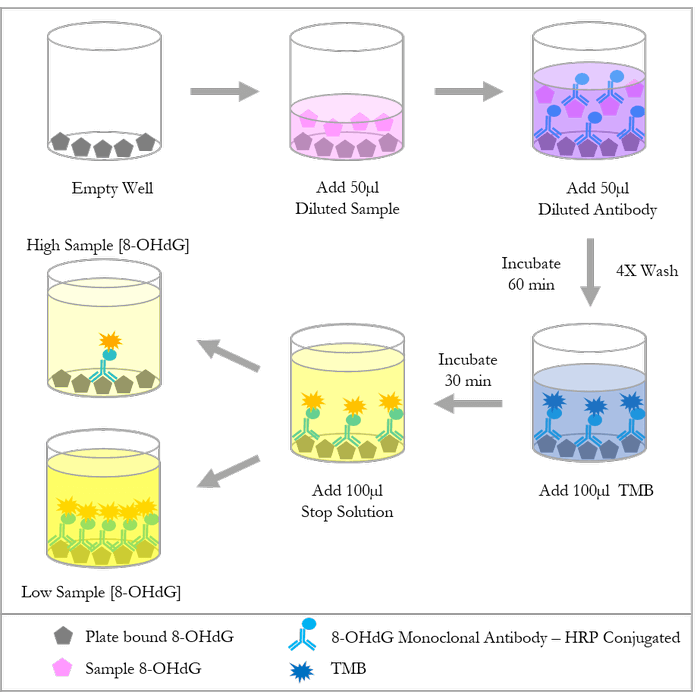
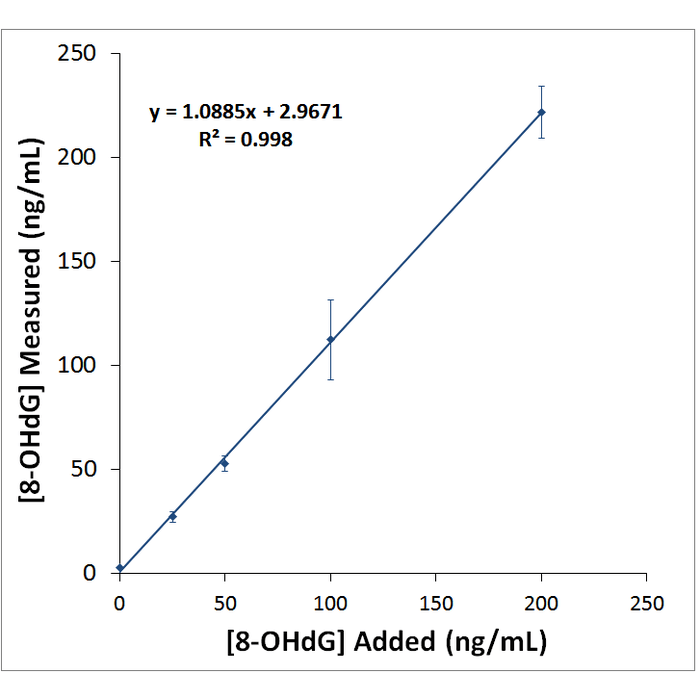
| Assay Overview | 1. Prepare standard and samples in the Sample and Standard Diluent. 2. Add 50 µL of prepared standards and samples in triplicate to appropriate wells. 3. Add 50 µL of the diluted antibody preparation to the appropriate wells. 4. Cover plate with Plate Cover and incubate at room temperature (20-25°C) for 1 hour. 5. Wash plate 4 times with 1X Wash Buffer. 6. Add 100 µL of TMB Substrate to each well. 7. Cover plate and develop the plate in the dark at room temperature for 30 minutes. 8. Add 100 µL of Stop Solution to each well. 9. Measure absorbance on a plate reader at 450 nm. 10. Plot the standard curve and calculate sample concentrations. | ||||||||||||||||||||||||||||||
| Kit Overview |
|
||||||||||||||||||||||||||||||
| Cite This Product | DNA Damage (8-OHdG) ELISA Kit (StressMarq Biosciences, Canada, Cat # SKT-120) |
Biological Description
| Alternative Names | 8 hydroxyguanine, 8-OH-dG, 8-OHdG, 80G, 8OHG, DNA Damage |
| Research Areas | Cancer, Cell Signaling, DNA Damage and Repair, DNA/RNA, Epigenetics and Nuclear Signaling, Oxidation, Oxidative Stress, Post-translational Modifications |
| Scientific Background |
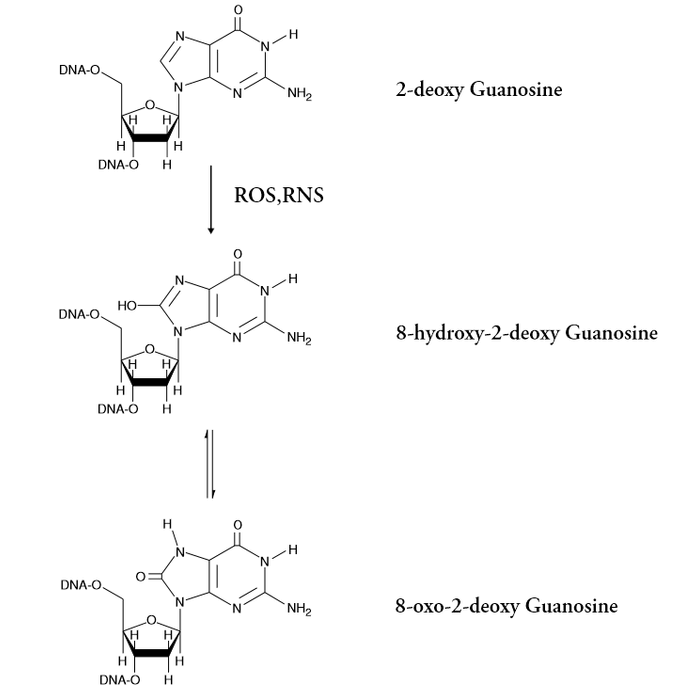
8-hydroxy-2'-deoxyguanosine (8-OHdG) is a widely recognized biomarker of oxidative DNA damage, formed when reactive oxygen and nitrogen species modify guanine bases. This hydroxylation process occurs during normal metabolism and is amplified by environmental stressors that increase oxidative load. Elevated levels of 8-OHdG are strongly associated with aging and a range of chronic conditions, including cancer, diabetes, hypertension, and neurodegenerative diseases. In neuroscience, 8-OHdG serves as a critical indicator of oxidative stress in brain tissue, where neurons are particularly vulnerable due to high metabolic activity and lipid content. Increased 8-OHdG levels have been linked to the progression of Alzheimer’s, Parkinson’s, and other neurodegenerative disorders, making it a valuable tool for monitoring disease onset and therapeutic response. 8-OHdG can exist as a free nucleoside or be incorporated into DNA. In biological samples such as plasma, cell lysates, and tissues, its detection is complex; however, urine provides a more reliable matrix for measuring free 8-OHdG due to efficient renal filtration. Typical urinary concentrations range from 2.7–13 ng/mg creatinine, while plasma levels are significantly lower, often between 4–21 pg/mL as measured by LC-MS. By tracking 8-OHdG levels, researchers gain insight into cellular oxidative damage, enabling early detection and evaluation of antioxidant-based interventions in neurodegenerative disease research. |
| References |
1. Maxey K.M., Maddipati K.R., Birkmeier J. (1992) J Clin Immunoassay 15: 116-120. 2. Pradelles P., Grassi J., Maclouf J. (1990) Methods Enzymol. 187: 24-34. 3. Maclouf J., Grassi J., Pradelles P. (1987) Dev Immunoassay Tech Meas eicosanoids. 4. Lin H., et al. (2004) Biochem J. 380: 541-548. 5. Bogdanov M.B., et al. (1999) Free Radic Biol Med. 27(5/6): 647-666. 6. Lee J., et al. (2005) Hypertension 45: 986-990. 7. Leinonen, J., et al. (1997) FEBSLett. 417: 150-152. 8. Endo K., et al. (2006) J. Atheroscler. Thromb. 13:68-75. 9. Kuo H., et al. (2007) Mutat Res. 631:62-68. 10. Shen J., et al. (2007) Cancer 109: 574-580. 11. Beckman K.B., Ames B.N. (1997) J Biol Chem 272: 19633-19636. 12. Epe B., et al. (1996) Nucleic Acids Res 24: 4105-4110. 13. Spencer J.P.E., et al. (1995) FEBS Lett 374: 233-236. 14. Floyd R.A. (1990) FASEB J 4: 2587-2597. |



StressMarq Biosciences :
Based on validation through cited publications.