Properties
| Storage Buffer | 20mM Hepes pH 7.4, 150mM NaCl, 1mM TCEP pH 7.0 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant Alpha Synuclein A90C Mutant Monomers (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SPR-478) |
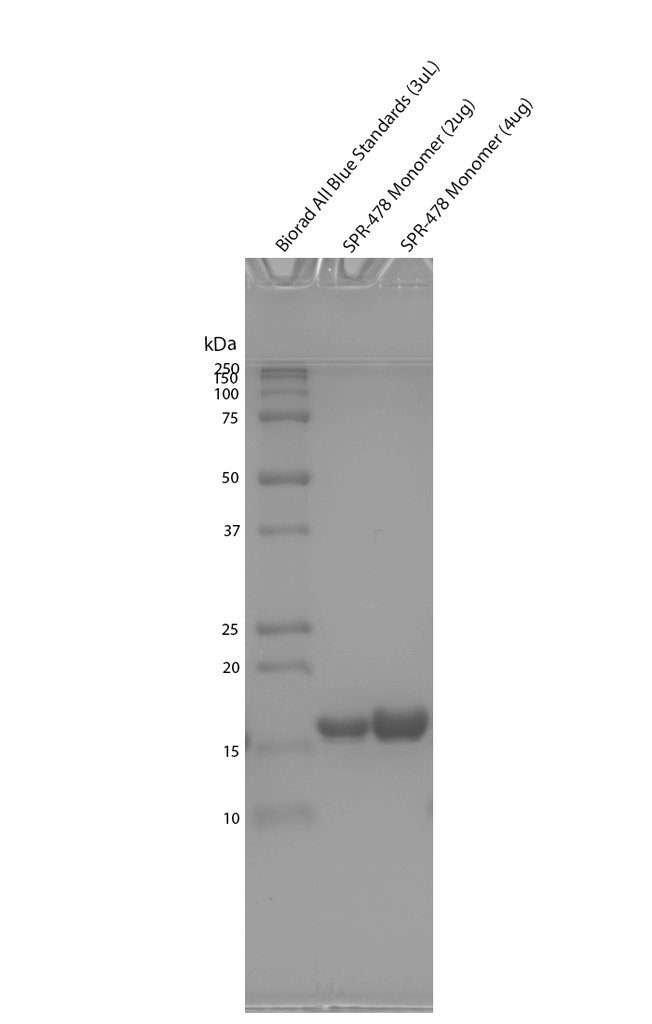
| Certificate of Analysis | Protein certified >95% pure on SDS-PAGE & Nanodrop analysis |
| Other Relevant Information | TCEP present to prevent disulfide bonds. Maleimide coupling reactions can be performed efficiently in the presence of TCEP. |
Biological Description
| Alternative Names | SNCA, alpha-synuclein, synuclein, Alpha synuclein monomer, Alpha-synuclein monomer, Alpha synuclein protein monomer, Alpha synuclein monomer, Alpha-synuclein protein, SNCA protein |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Parkinson's Disease, Synuclein, Tangles & Tau, Multiple System Atrophy |
| Swiss Prot | P37840-1 (wildtype) |
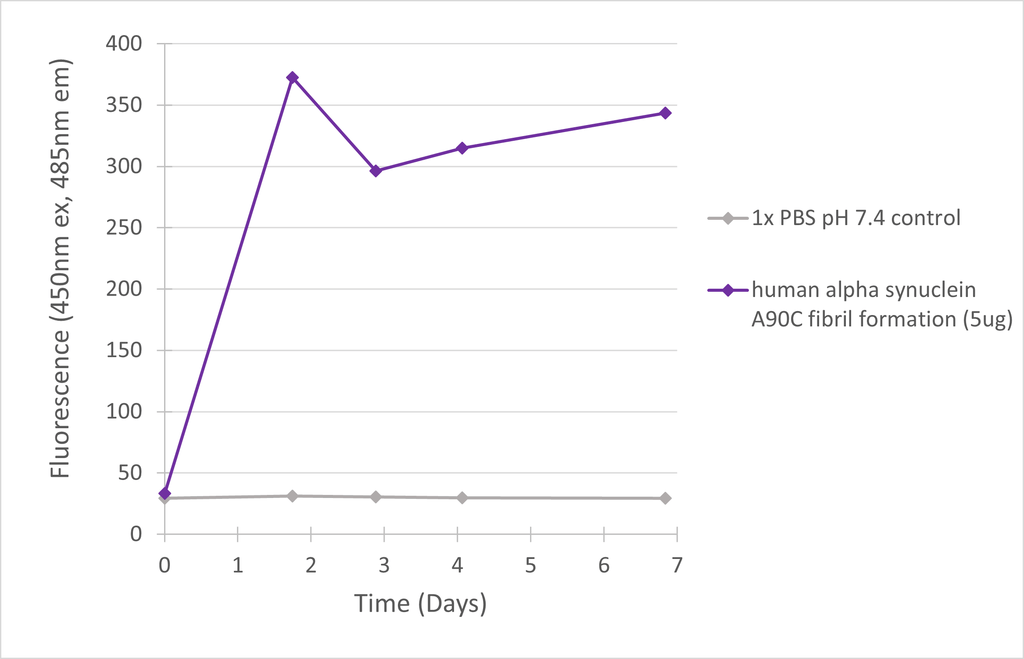
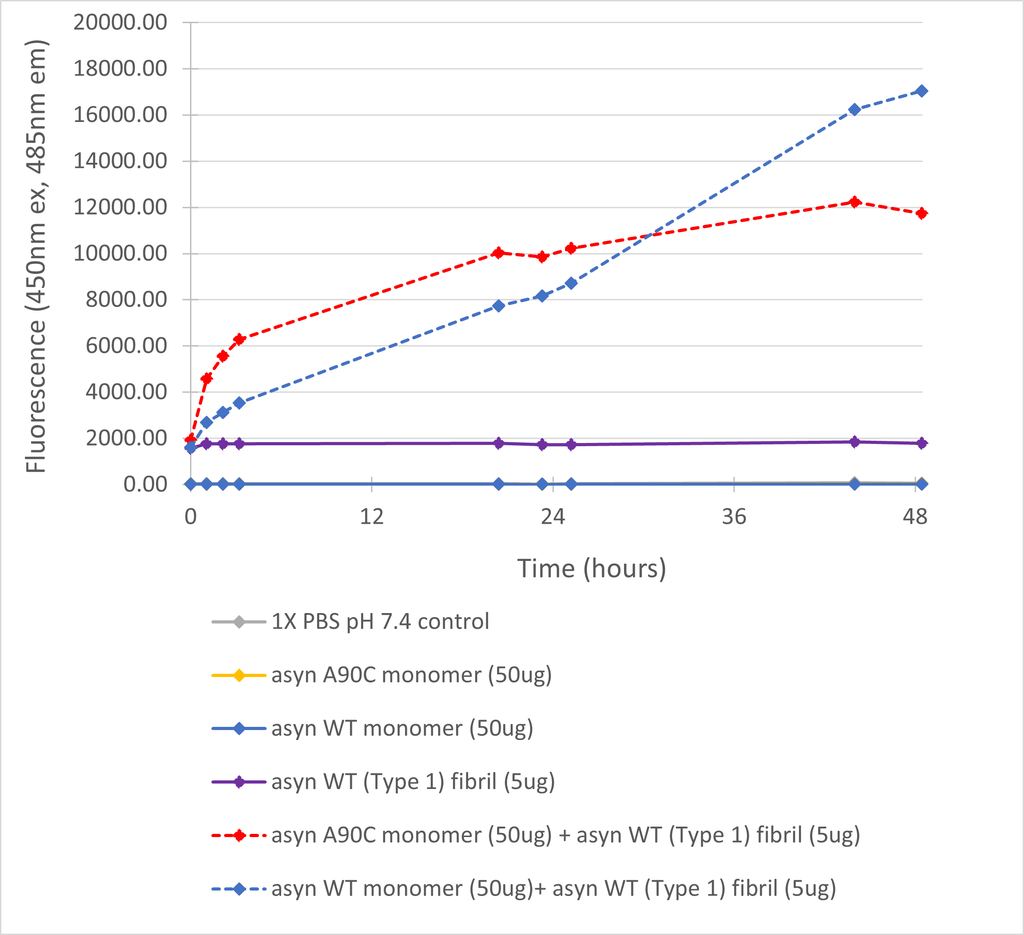
| Scientific Background | Thioflavin-T (ThT) fluorescence remains a common measurement of alpha-synuclein fibril formation, yet ThT exhibits poor affinity for oligomers and early aggregates. The alpha-synuclein A90C mutant monomers can be specifically labelled with alternative fluorophores (such as Alexa 488/Alexa 647) via maleimide chemistry to enable more sensitive FRET analysis of aggregation. The A90C mutant showed no perturbation of monomer structure and Alexa Fluor dye attachment to cysteine 90 was demonstrated to have no effect on the kinetics of fibril formation (1-3). Residue 90 is at the periphery of the NAC region, a key constituent of the alpha-synuclein β-sheet fibril core, which results in fluorophores on different monomers coming into close proximity upon formation of β-sheet structure during aggregation (4). Note - to prevent the potential mis-translation of alpha-synuclein Y136 as C136 during E.coli expression, the Y136-TAT construct was used (5). |
| References |
1.Thirunavukkuarasu, et al. 2008. Multiparametric Flurorescence Detection of Early Stages in the Amylord Protein Aggregation of Pyrene-labeled α-Synuclein. J. Mol. Biol. 378(5): 1064-73. https://doi.org/10.1016/j.jmb.2008.03.034 2.Cremades, et al. 2012. Direct Observation of the Interconversion of Normal and Toxic Forms of α-Synuclein. Cell. 149(5): 1048-59. doi: 10.1016/j.cell.2012.03.037 3.Horrocks et al. 2015. Fast Flow Microfluidics and Single-Molecule Fluorescence for the Rapid Characterization of α-Synuclein Oligomers. Anal. Chem. 87(17): 8818-26. https://doi.org/10.1021/acs.analchem.5b01811 4.Iljina et al. 2016. Kinetic model of the aggregation of alpha-synuclein provides insights into prion-like spreading. PNAS. 113(19): E1206-15. doi: 10.1073/pnas.1524128113 5.Masuda, et al. 2006. Cysteine misincorporation in bacterially expressed human alpha-synuclein. FEBS Lett. 580(7): 1775-9. doi: 10.1016/j.febslet.2006.02.032 |
Product Images
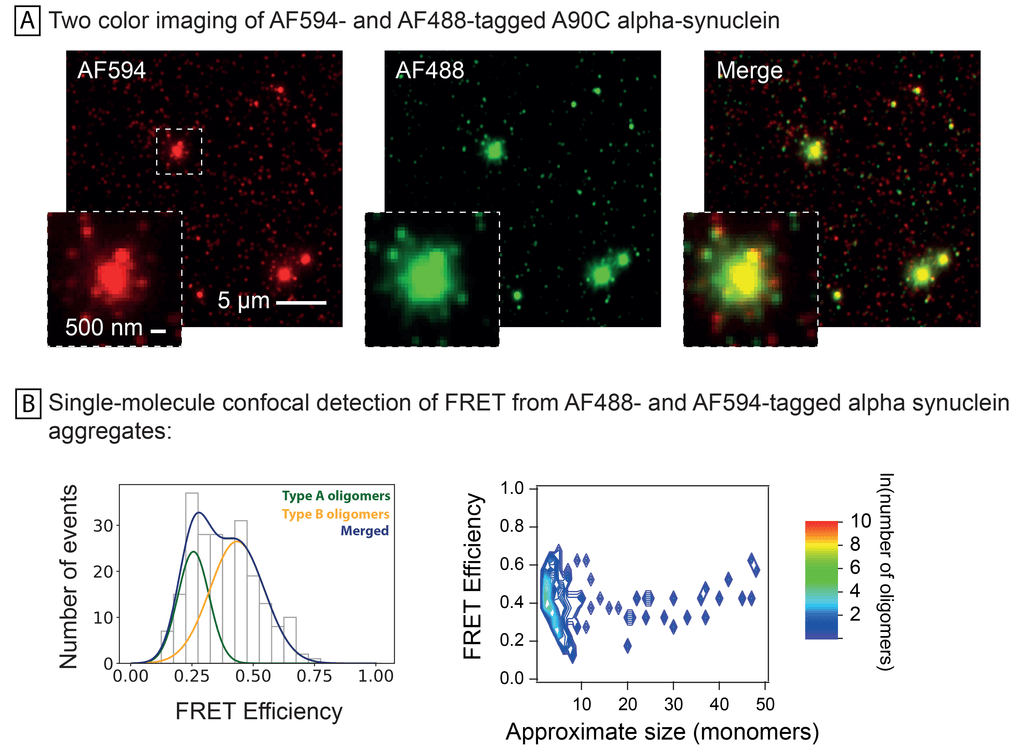
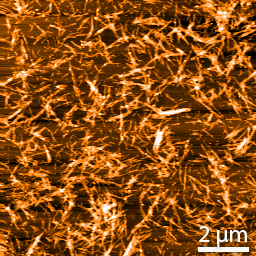
Co-aggregation of AF594 and AF488-labelled A90C alpha-synuclein monomers. Half of monomers were labelled with AF594 and half with AF488, and then allowed to aggregate prior to imaging using (A) total internal reflection microscopy (TIRFM) at the two excitation and emission maxima and (B) single-molecule confocal FRET detection of two oligomer types at Ex488 and Em594. In depth method and oligomer details were previously described by Horrocks et al. in 2015 (https://doi.org/10.1021/acs.analchem.5b01811).





















Reviews
There are no reviews yet.