Properties
| Storage Buffer | 10mM PB pH 7.4, 10mM DTT |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange, ammonium sulfate precipitation and SEC purified |
| Cite This Product | Human Recombinant Tau dGAE (297-391) Monomers (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SPR-501) |
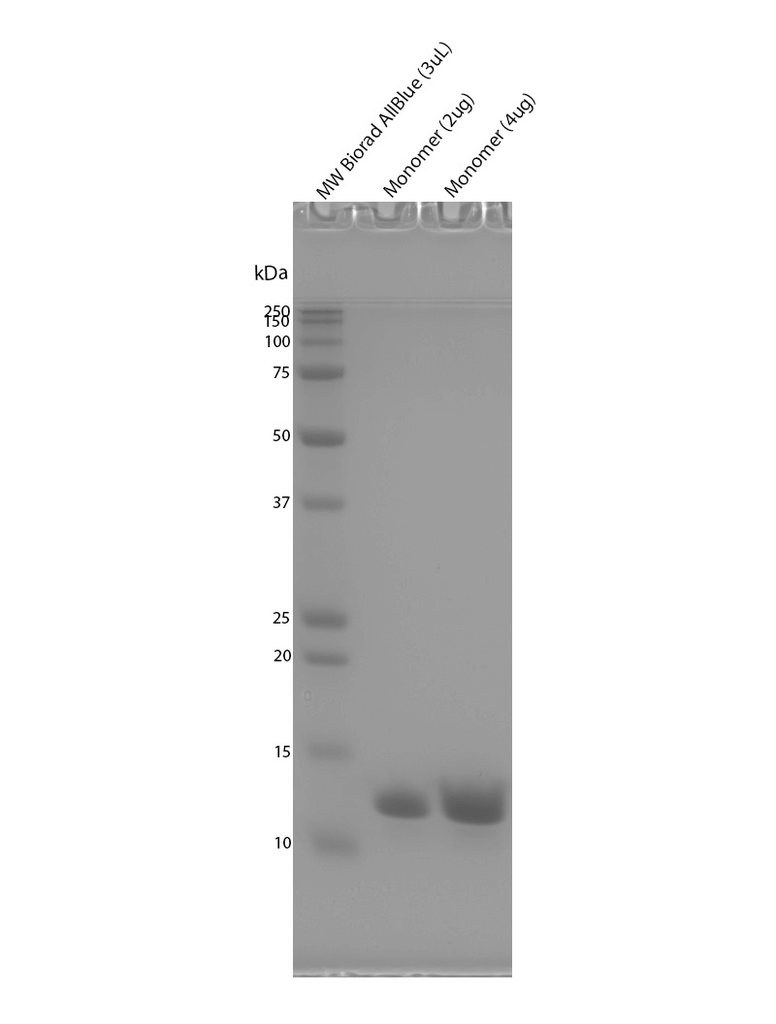
| Certificate of Analysis | Protein certified >95% pure on SDS-PAGE & Nanodrop analysis. Low endotoxin <5 EU/mL @ 2mg/mL. |
| Other Relevant Information | For corresponding PFFs, see catalog# SPR-502 |
Biological Description
| Alternative Names | Tau monomer, Tau protein monomer, Tau protein, microtubule-associated protein Tau, MAPT, MAP, microtubule-associated protein, Truncated Tau Protein Monomer, Paired Helical Filament-Tau, Phf-Tau, Neurofibrillary Tangle Protein, Tau dGAE Protein, dGAE |
| Research Areas | Alzheimer's Disease, Axon Markers, Cell Markers, Cell Signaling, Cytoskeleton, Microtubules, MT Associated Proteins, Neurodegeneration, Neuron Markers, Neuroscience, Tangles & Tau |
| Swiss Prot | P10636-8 |
| Scientific Background | Filamentous tau inclusions are a hallmark of many neurodegenerative diseases, including Alzheimer’s disease (AD) and Chronic Traumatic Encephalopathy (CTE), collectively called tauopathies. Advances in Cryo-EM have revealed that tau filaments isolated from individuals with a particular neurodegenerative disease share a distinct tau fold – i.e. an AD-isolated Tau filaments’ fold is distinct from a CTE-isolated Tau filaments’ fold (1-3). Utilizing Tau filaments with the correct disease-specific fold is an important goal towards better mimicking specific human diseases in cellular and in vivo models. Recent Cryo-EM studies have demonstrated that recombinantly generated Tau dGAE monomers will form the disease-isolated AD or CTE Tau filament folds under highly specific conditions in vitro (4, 5). StressMarq’s SPR-501 Tau (297-391) dGAE monomers are purified following these exact published procedures and can be utilized to form these distinct folds using specific aggregation conditions. |
| References |
1. Goedert, Eisenberg and Crowther. 2017. Propagation of Tau Aggregates and Neurodegeneration. Annu Rev Neurosci. DOI: https://doi.org/10.1146/annurev-neuro-072116-031153 2. Fitzpatrick et al. 2017. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. DOI: 10.1038/nature23002 3. Falcon et al. 2019. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. DOI: https://doi.org/10.1038/s41586-019-1026-5. 4. Lovestam et al. 2022. Assembly of Recombinant Tau into Filaments Identical to those of Alzheimer’s disease and Chronic Traumatic Encephalopathy. eLife. DOI: https://doi.org/10.7554/eLife.76494 5. Lovestam et al. 2023. Disease-specific Tau Filaments Assemble via Polymorphic Intermediates. bioRxiv. https://doi.org/10.1101/2023.07.24.550295 |
Product Images
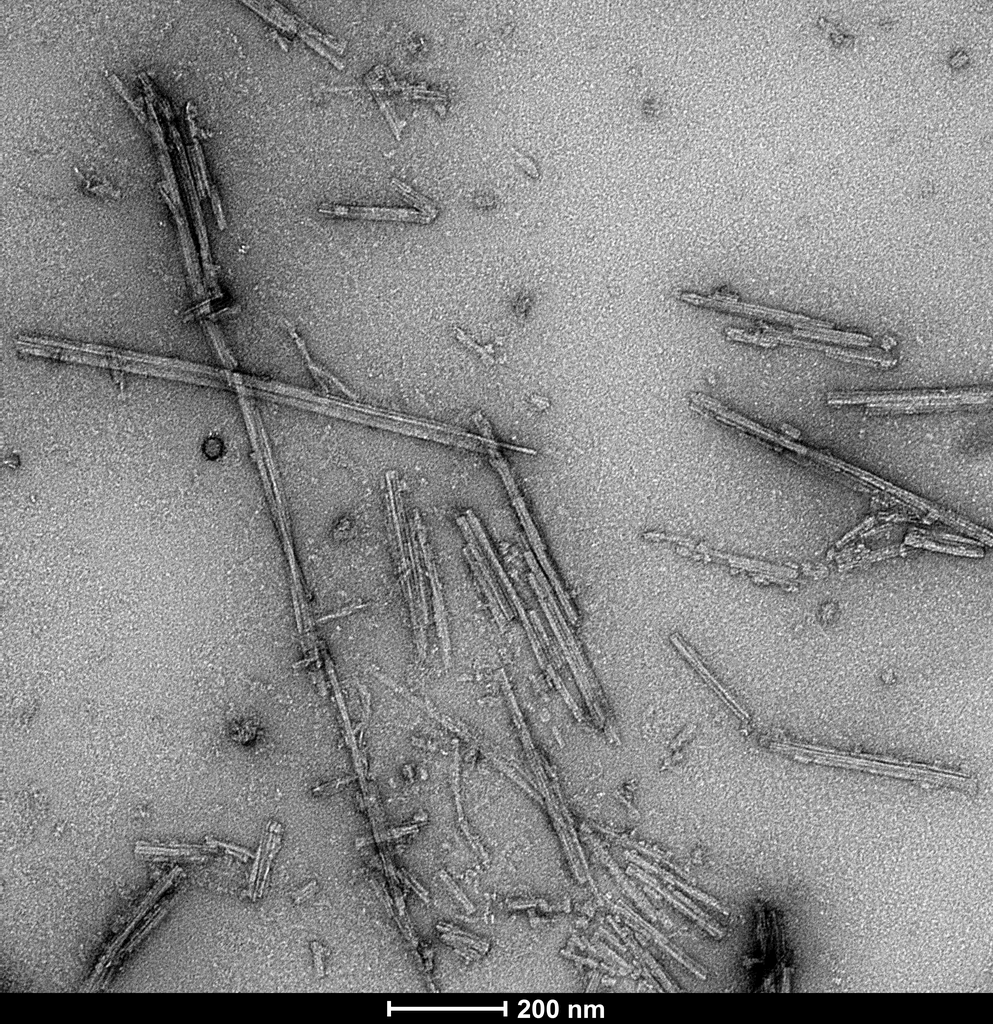
TEM of Tau dGAE AD-mimic fibrils (SPR-502) generated from Tau dGAE monomers (SPR-501) by shaking 200 rpm at 37oC for 48 hours in 10 mM PB 10 mM DTT pH 7.4 with 200 mM MgCl2 added (Lovestam et al. 2022, eLife). Negative stain transmission electron microscopy images acquired at 80 Kv on carbon coated 400 mesh copper grids using phosphotungstic acid and uranyl acetate stain. Scale bar = 200 nm.





















Reviews
There are no reviews yet.