Properties
| Storage Buffer | PBS pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant TTR Protein (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog #SPR-452 ) |
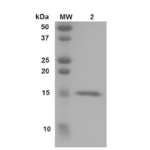
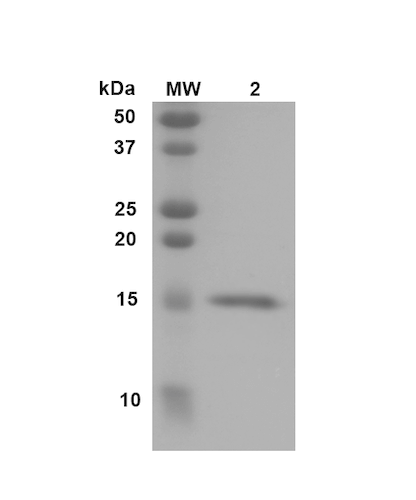
| Certificate of Analysis | Certified >95% pure using SDS-PAGE analysis. Low endotoxin <5 EU/mL @ 2mg/mL. |
| Other Relevant Information | For corresponding PFFs, see catalog# SPR-465 |
Biological Description
| Alternative Names | Amyloid polyneuropathy Protein Monomer, Amyloidosis I Protein Monomer, ATTR Protein Monomer, Carpal tunnel syndrome 1 Protein Monomer, CTS Protein Monomer, CTS1 Protein Monomer, HEL111 Protein Monomer, HsT2651 Protein Monomer, PALB Protein Monomer, Prealbumin Protein Monomer, Prealbumin amyloidosis type I Protein Monomer, Prealbumin Thyroxine-binding Protein Monomer, TBPA Protein Monomer, Thyroxine binding prealbumin Protein Monomer, Transthyretin Protein Monomer, TTHY_HUMAN Protein Monomer, TTR Protein Monomer, TTR protein |
| Research Areas | ALS Disease, Alzheimer's Disease, Blood, Cardiovascular System, Cell Signaling, Lipid and lipoprotein Metabolism, Metabolism, Neurodegeneration, Neuroscience, Parkinson's Disease, Tangles & Tau |
| Cellular Localization | Cytoplasm, Extracellular exosome, Extracellular Region, Lysosome |
| Accession Number | NP_000362.1 |
| Gene ID | 7276 |
| Swiss Prot | P02766 |
| Scientific Background | Transthyretin is a transport protein in the serum and cerebospinal fluid that carried the thyroid hormone Thyroxine and retinol-binding protein bound to retinol. TTR misfolding and aggregation is known to be associated with the amyloiddiseases SSA, FAP and FAC (1-5). TTR is also thought to have beneficial side effects, such as binding to beta-amyloid protein, preventing beta-amyloid from accumulating into the plaques associated with Alzheimer's Disease (6). The mutant variant Y78F indicates a destabilization of the contacts between the alpha-helix and AB loop and the body of the molecule, potentially leading to applications in immne therapy for FAP (7). |
| References |
1. Zeldenrust S.R., Benson M.D. (2010). Wiley. pp. 795–815. 2. Westermark P., Sletten K., Johansson B., Cornwell G.G. (1990). Proc. Natl. Acad. Sci. U.S.A. 87(7): 2843–5. 3. Andrade C. (1952). Brain. 75(3): 408–27. 4. Coelho T. (1996). Curr. Opin. Neurol. 9(5): 355–9. 5. Jacobson D.R, et. al. (1997). N. Engl. J. Med. 336(7): 466–73. 6. Li X. (2011). Mol Neurodegener. 6(1):79. 7. Terazaki H.,m et al. (2006) Lab Invest. 86(1): 23-31. |





















Reviews
There are no reviews yet.