| Product Name | AP-18 |
| Description |
TRPA1 blocker |
| Purity | >98% |
| CAS No. | 55224-94-7 |
| Molecular Formula | C11H12ClNO |
| Molecular Weight | 209.67 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol |
| Source | Synthetic |
| Appearance | White Solid |
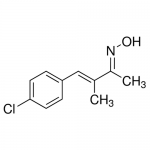
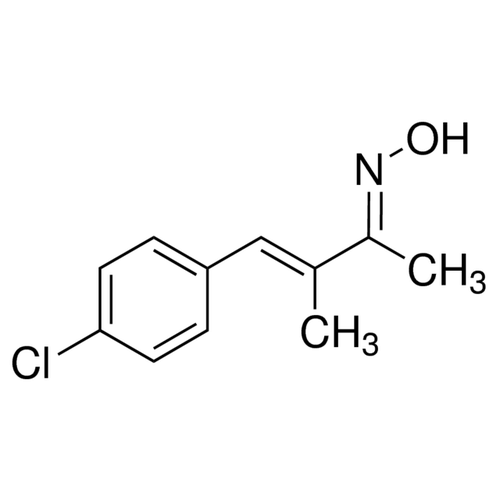
| SMILES | C/C(=CC1=CC=C(C=C1)Cl)/C(=N/O)/C |
| InChI | InChI=1S/C11H12ClNO/c1-8(9(2)13-14)7-10-3-5-11(12)6-4-10/h3-7,14H,1-2H3/b8-7-,13-9+ |
| InChIKey | MHTJEUOFLVQMCL-XTYDYKDCSA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection Hazard Phrases: H302 |
| Cite This Product | AP-18 (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-310) |
Biological Description
| Alternative Names | (2E,3E)-4-(4-Chlorophenyl)-N-hydroxy-3-methyl-3-buten-2-imine |
| Research Areas | Ion Channels, Neuroscience |
| PubChem ID | 16725814 |
| Scientific Background | AP-18 is a reversible antagonist of the TRPA1 ion channel, which is involved in sensing noxious stimuli and mediating pain and inflammation. In neuroscience, AP-18 is used to study TRPA1’s role in neurogenic inflammation, neuropathic pain, and sensory neuron excitability. By blocking TRPA1, AP-18 helps delineate the molecular mechanisms underlying pain perception and may contribute to the development of novel analgesics targeting TRP channels. |
| References | 1. Doi H., et al. (2005) Tohoku J Exp Med. 207(3): 209-216. |

Reviews
There are no reviews yet.