| Product Name | Amiodarone Hydrochloride |
| Description |
Autophagy inducer |
| Purity | >99% |
| CAS No. | 19774-82-4 |
| Molecular Formula | C25H29I2NO3•HCl |
| Molecular Weight | 681.77 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inducer |
| Solubility | Soluble to 50 mM in DMSO |
| Source | Synthetic |
| Appearance | White solid |
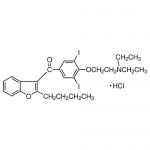
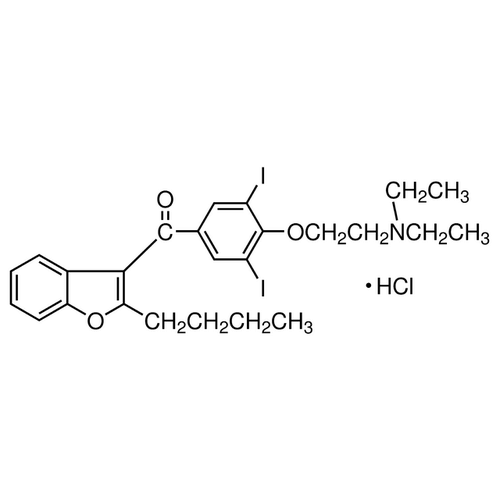
| SMILES | [H+].C1=CC=CC2=C1C(=C(O2)CCCC)C(=O)C3=CC(=C(OCCN(CC)CC)C(=C3)I)I.[Cl-] |
| InChI | InChI=1S/C25H29I2NO3.ClH/c1-4-7-11-22-23(18-10-8-9-12-21(18)31-22)24(29)17-15-19(26)25(20(27)16-17)30-14-13-28(5-2)6-3;/h8-10,12,15-16H,4-7,11,13-14H2,1-3H3;1H |
| InChIKey | ITPDYQOUSLNIHG-UHFFFAOYSA-N |
| Safety Phrases |
Classification: D1B- Toxic Material Causing Immediate and Serious, Toxic Effects, Toxic by inhalation. Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. Hazard statements: H303- May be harmful if swallowed. H312 + H332- Harmful in contact with skin or if inhaled Precautionary statements: P280- Wear protective gloves/ protective clothing. |
| Cite This Product | Amiodarone Hydrochloride (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-393) |
Biological Description
| Alternative Names | 2-Butyl-3-benzofuranyl-4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone hydrochloride |
| Research Areas | Autophagy, Cancer |
| PubChem ID | 441325 |
| Scientific Background | Amiodarone Hydrochloride is a non-selective ion channel blocker primarily used as an antiarrhythmic agent. In neuroscience, Amiodarone has been shown to induce autophagy by inhibiting mTORC1, a key regulator of cellular metabolism and protein turnover. In yeast models, it triggers calcium influx, mitochondrial fragmentation, and cell death—mechanisms that parallel neurodegenerative processes. Amiodarone’s ability to modulate autophagy and mitochondrial dynamics makes it a useful compound for studying neurodegeneration, particularly in models of Parkinson’s disease and other disorders involving mitochondrial dysfunction. Its broad-spectrum activity also supports research into ion channel regulation and calcium signaling in neuronal cells. |
| References |
1. Zhang Y., et al. (2007). J Biol Chem. 282(52): 37844-53. 2. Balgi A., et al. (2009). PLoS One. 4(9): e7124. |

Reviews
There are no reviews yet.