| Product Name | Chaetocin |
| Description |
Methyltransferase inhibitor |
| Purity | >99% (TLC), NMR conforms |
| CAS No. | 28097-03-2 |
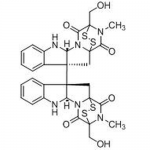
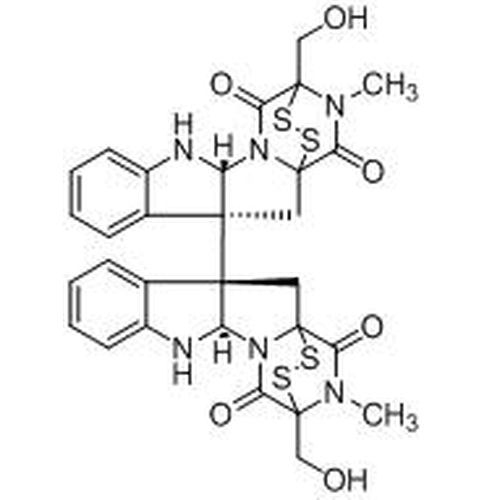
| Molecular Formula | C30H28N6O6S4 |
| Molecular Weight | 696.9 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in 25 mg/ml DMSO or 25 mg/ml Ethanol |
| Source | Synthetic |
| Appearance | Lyophilized Solid |
| InChI | InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40 |
| InChIKey | PZPPOCZWRGNKIR-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Harmful- Harmful by inhalation, in contact with skin, and if swallowed. Safety Phrases: S22 - Do not breathe dust S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S24/25- Avoid contact with skin and eyes Precautionary Statements: P280 Wear protective gloves/protective clothing/eye protection/face protection. P301+P312- IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell. P304+P340- IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. P501 Dispose of contents/container in accordance with local/regional/national/international regulations. Hazard Statements: H302- Harmful if swallowed H312- Harmful in contact with skin H332- Harmful if inhaled |
| Cite This Product | Chaetocin (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-351) |
Biological Description
| Alternative Names | 2,2',3S,3'S,5aR,5'aR,6,6'-octahydro-3,3'-bis(hydroxymethyl)-2,2'-dimethyl-[10bR,10'bR(11aS,11'aS)-bi-3,11a-epidithio-11aH-pyrazino[1',2':1,5]pyrrolo[2,3-b]indole]-1,1',4,4'-tetrone |
| Research Areas | Cancer, Cell Signaling |
| PubChem ID | 161591 |
| Scientific Background | Chaetocin is a fungal-derived inhibitor of the lysine methyltransferase SUV39H1, a key enzyme involved in histone H3 lysine 9 (H3K9) methylation. While primarily studied for its antimyeloma and anticancer properties, Chaetocin has gained attention in neuroscience for its role in epigenetic regulation. By modulating histone methylation, Chaetocin influences gene expression patterns critical to neuronal development, synaptic plasticity, and memory formation. Its ability to alter chromatin structure makes it a valuable tool for investigating the epigenetic mechanisms underlying neurodegenerative diseases such as Alzheimer's and Huntington's. Chaetocin’s cell permeability and specificity for SUV39H1 position it as a promising candidate for exploring therapeutic strategies targeting aberrant histone methylation in the brain. |
| References |
1. Greiner D., et al. (2005) Nat Chem Biol. 1: 143. 2. Isham, C.R., et al. (2007) Blood. 109: 2579. |

StressMarq Biosciences :
Based on validation through cited publications.