| Product Name | Entacapone |
| Description |
COMT inhibitor |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 130929-57-6 |
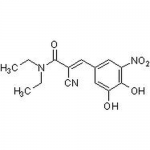
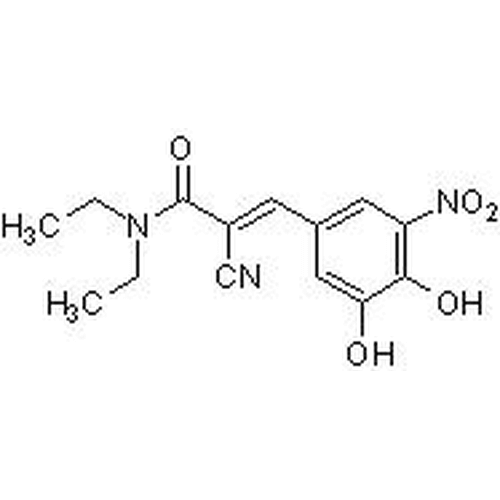
| Molecular Formula | C14H15N3O5 |
| Molecular Weight | 305.3 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (30 mg/ml); or ethanol (3 mg/ml) |
| Source | Synthetic |
| Appearance | Yellow powder |
| SMILES | OC1=CC(/C=C(C#N)/C(N(CC)CC)=O)=CC([N+]([O-])=O)=C1O |
| InChI | InChI=1S/C14H15N3O5/c1-3-16(4-2)14(20)10(8-15)5-9-6-11(17(21)22)13(19)12(18)7-9/h5-7,18-19H,3-4H2,1-2H3/b10-5+ |
| InChIKey | JRURYQJSLYLRLN-BJMVGYQFSA-N |
| Safety Phrases |
Classification: Not a hazardous substance or mixture. Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. |
| Cite This Product | Entacapone (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-578) |
Biological Description
| Alternative Names | (2E)-2-Cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-propenamide |
| Research Areas | Cell Signaling, Neurodegeneration, Neuroscience, Neurotransmission, Parkinson's Disease, Synuclein |
| PubChem ID | 5281081 |
| Scientific Background |
Entacapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT), an enzyme responsible for the peripheral degradation of levodopa. Clinically, it is used as an adjunct therapy in Parkinson’s disease to prolong the half-life and efficacy of levodopa by preventing its premature metabolism. Beyond its established role in dopaminergic modulation, Entacapone has demonstrated potential neuroprotective properties relevant to neurodegenerative disease research. Recent studies suggest that Entacapone may inhibit the oligomerization and fibrillogenesis of alpha-synuclein and beta-amyloid—two pathological proteins central to the progression of Parkinson’s and Alzheimer’s diseases, respectively. By interfering with the aggregation of these proteins, Entacapone may reduce neurotoxicity and synaptic dysfunction, offering a dual mechanism of action: symptomatic relief through dopaminergic support and disease modification through anti-aggregation effects. Additionally, its modulation of catecholamine metabolism may influence oxidative stress and neuroinflammation, both of which are implicated in the pathophysiology of neurodegeneration. These emerging findings position Entacapone as a compound of interest not only in symptomatic management but also in the exploration of disease-modifying strategies for neurodegenerative disorders. |
| References | 1. Di Giovanni S., et al. (2010) J Biol Chem. 285 (20): 14941-14954. |

Reviews
There are no reviews yet.