| Product Name | Galantamine Hydrobromide |
| Description |
Cholinesterase Inhibitor |
| Purity | >97% (HPLC); NMR (Conforms) |
| CAS No. | 1953-04-4 |
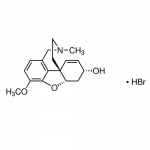
| Molecular Formula | C17H22BrNO3 |
| Molecular Weight | 368.3 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Blue Ice or 4ºC |
| Product Type | Inhibitor |
| Solubility | Soluble in water (12 mg/ml) or in DMSO (10 mg/ml) |
| Source | Synthetic |
| Appearance | White powder |
| SMILES | [Br-].COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24.[H+] |
| InChI | 1S/C17H21NO3.BrH/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17;/h3-6,12,14,19H,7-10H2,1-2H3;1H/t12-,14-,17-;/m0./s1 |
| InChIKey | QORVDGQLPPAFRS-XPSHAMGMSA-N |
| Safety Phrases |
Classification: D1B Toxic Material Causing Immediate and Serious Toxic Effects - Toxic by ingestion Hazard statement(s): H301 Toxic if swallowed. Precautionary statement(s): P301 + P310 IF SWALLOWED: Immediately call a POISON CENTER or doctor/ physician. |
| Cite This Product | Galanthamine HBr (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-530) |
Biological Description
| Alternative Names | Galantamine hydrobromide, Galantamine HBr, Galanthamine hydrobromide, Galanthamine, Galanthamine hydrogen bromide, SPECTRUM1501202, 1,2,3,4,6,7,7a,11c-Octahydro-9-methoxy-2-methyl-benzofuro(4,3,2-efg)(2)benzazocin-6-ol HBr, LS-185021, Galanthamine, Nivalin, Reminyl, Jilkon hydrobromide, Lycoremine hydrobromide |
| Research Areas | Alzheimer's Disease, Apoptosis, Cancer, Neurodegeneration, Neuroscience |
| PubChem ID | 121587 |
| Scientific Background | Galanthamine hydrobromide is a long-acting acetylcholinesterase inhibitor and an allosteric potentiator of nicotinic acetylcholine receptors. It is widely used in the treatment of Alzheimer’s disease due to its ability to enhance cholinergic neurotransmission. Galanthamine also exhibits neuroprotective effects by preventing β-amyloid-induced apoptosis and reducing amyloid precursor protein deposition. Its dual mechanism of action makes it a valuable compound in neurodegenerative disease research and cognitive enhancement studies. |

Reviews
There are no reviews yet.