| Product Name | Herbimycin A |
| Description |
Hsp90 inhibitor |
| Purity | >98% |
| CAS No. | 70563-58-5 |
| Molecular Formula | C30H42N2O9 |
| Molecular Weight | 574.3 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in DMSO (>25 mg/ml) and ethanol (10 mg/ml) |
| Source | Synthetic |
| Appearance | Yellow Solid |
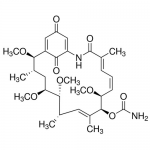
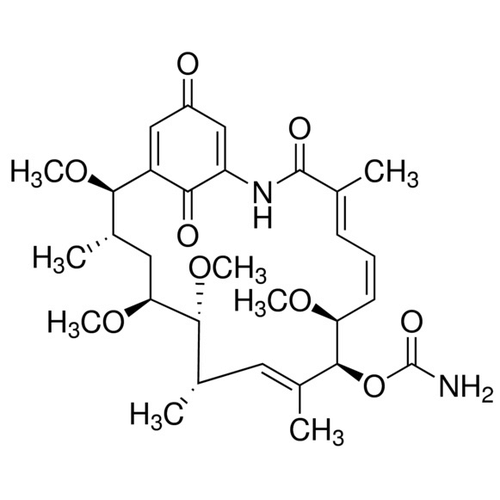
| SMILES | C[C@H]1C[C@@H]([C@@H]([C@H](/C=C(/[C@@H]([C@H](/C=CC=C(C(=O)NC2=CC(=O)C=C([C@@H]1OC)C2=O)/C)OC)OC(=O)N)C)C)OC)OC |
| InChI | InChI=1S/C30H42N2O9/c1-16-10-9-11-23(37-5)28(41-30(31)36)18(3)12-17(2)27(40-8)24(38-6)13-19(4)26(39-7)21-14-20(33)15-22(25(21)34)32-29( |
| InChIKey | MCAHMSDENAOJFZ-BVXDHVRPSA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection |
| Cite This Product | Herbimycin A (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-116) |
Biological Description
| Alternative Names | [(2R,3S,5S,6R,7S,8E,10S,11S,12E,14E)-2,5,6,11-tetramethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate |
| Research Areas | Cancer, Heat Shock |
| PubChem ID | 6436247 |
| Scientific Background | Herbimycin A is a benzoquinoid ansamycin antibiotic known for its irreversible inhibition of tyrosine kinases through thiol group interaction. While predominantly studied in oncology, Herbimycin A’s ability to modulate kinase signaling cascades positions it as a candidate for neurodegenerative disease research. Tyrosine kinases play critical roles in neuronal survival, synaptic plasticity, and neuroinflammation. By inhibiting kinases such as Src and Abl, Herbimycin A may help attenuate aberrant signaling associated with neurodegenerative conditions. Its potential to influence phospholipase C activity and downstream pathways further supports its relevance in neuroscience, particularly in the context of cellular stress responses and neuroprotection. |
| References |
1. Uehara Y., and Fukazawa H. (1991) Methods Enzymol. 201: 370. 2. Fukazawa H., et al. (1991) Biochem. Pharmacol. 42: 1661. 3. Satoh T., et al. (1992) J.Biol.Chem. 267: 2537. 4. Weiss R., and Nuccitelli R. (1992) J.Biol.Chem. 267:5608. |

Reviews
There are no reviews yet.