| Product Name | Nelfinavir mesylate |
| Description |
Potent HIV-1 protease inhibitor |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 159989-65-8 |
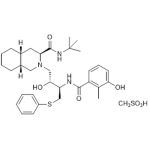
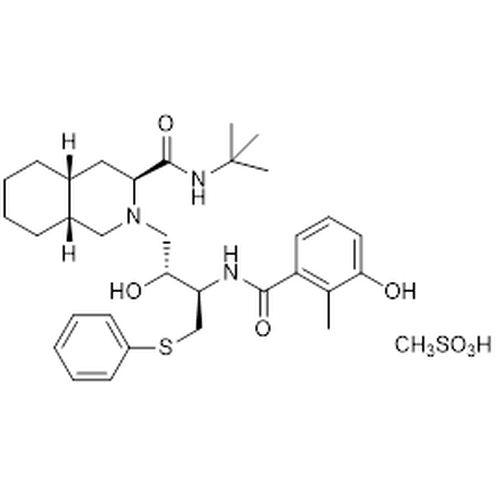
| Molecular Formula | C32H45N3O4S • CH3SO3H |
| Molecular Weight | 663.9 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (70 mg/ml) |
| Source | Synthetic |
| Appearance | White powder |
| SMILES | [H][C@]12CCCC[C@]([H])1C[C@@H]([C@@](NC(C)(C)C)=O)N(C[C@@H](O)[C@H](CSC3=CC=CC=C3)NC(C4=C(C)C(O)=CC=C4)=O)C2.CS(=O)(O)=O |
| InChI | InChI=1S/C32H45N3O4S.CH4O3S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4;1-5(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8 |
| InChIKey | NQHXCOAXSHGTIA-SKXNDZRYSA-N |
| Safety Phrases | Hazard Statement: H413 May cause long lasting harmful effects to aquatic life. |
| Cite This Product | Nelfinavir mesylate (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-586) |
Biological Description
| Alternative Names | (3S,4aS,8aS)-N-(1,1-Dimethylethyl)decahydro-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-3-isoquinolinecarboxamide methanesulfonate; AG-1343 |
| Research Areas | Apoptosis, Autophagy, Cancer |
| PubChem ID | 64142 |
| Scientific Background |
Nelfinavir mesylate, originally developed as an HIV protease inhibitor, has gained attention for its broader biological effects, including autophagy induction and anti-cancer activity. It modulates the Akt/PKB signaling pathway and triggers endoplasmic reticulum stress, leading to activation of the unfolded protein response. These mechanisms are increasingly relevant in neurodegenerative disease research, where protein misfolding, ER stress, and impaired autophagy are central features. Nelfinavir’s ability to influence these pathways positions it as a potential candidate for repurposing in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. |
| References | 1. Koltai T., (2015). F1000Res. 4(2): 9. 2. Yamamoto N., et al. (2004) Biochem Biophys Research Comm. 318: 719-725. |

Reviews
There are no reviews yet.