| Product Name | Staurosporine |
| Description |
Protein kinase inhibitor |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 62996-74-1 |
| Molecular Formula | C28H26N4O3 |
| Molecular Weight | 466.54 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 50 mM in DMSO |
| Source | Synthetic |
| Appearance | Pale Yellow Solid |
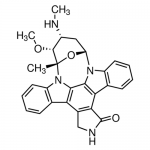
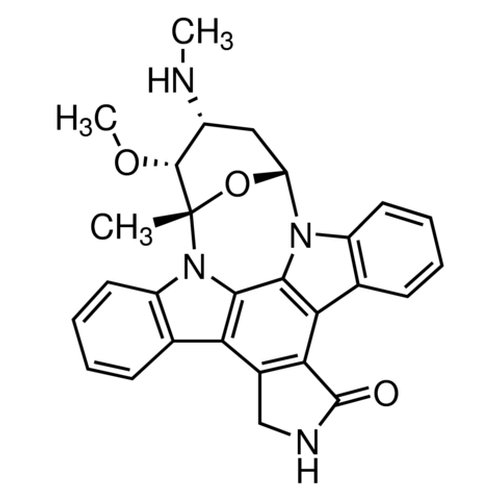
| SMILES | C[C@@]12[C@@H]([C@@H](C[C@@H](O1)N3C4=CC=CC=C4C5=C6C(=C7C8=CC=CC=C8N2C7=C53)CNC6=O)NC)OC |
| InChI | InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(2 |
| InChIKey | HKSZLNNOFSGOKW-FYTWVXJKSA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection Hazard Phrases: H340- H350 Precautionary Phrases: P201-P308 + P313 |
| Cite This Product | Staurosporine (StressMarq Biosciences, Canada, Cat # SIH-253) |
Biological Description
| Alternative Names | (2S,3R,4R,6R)-3-Methoxy-2-methyl-4-(methylamino)-29-oxa-1,7,17-triazaoctacyclo[12.12.2.12,6.07,28.08,13.015,19.020,27.021,26]nonacosa-8,10,12,14,19,21,23,25,27-nonaen-16-one |
| Research Areas | Apoptosis, Cancer, Cancer Growth Inhibitors, Tyrosine Kinase Inhibitors |
| PubChem ID | 44259 |
| Scientific Background | Staurosporine is a broad-spectrum protein kinase inhibitor with high affinity for ATP-binding sites of numerous kinases. It is widely used in neuroscience research to induce apoptosis and study cell death pathways. Staurosporine's ability to trigger caspase activation and mitochondrial dysfunction makes it a standard tool for modeling neurodegeneration in vitro. Despite its lack of selectivity, it remains a cornerstone compound for dissecting kinase-dependent signaling in neuronal cells. |
| References |
1. Ruegg U.T., and Burgess G.M. (1989) Trends in Pharm Science. 10(6): 218-220. 2. Karaman M.W., et al. (2008) Nat. Biotechnol. 26(1): 127-132. |

Reviews
There are no reviews yet.