| Product Name | Trichostatin A |
| Description |
HDAC inhibitor |
| Purity | >98% |
| CAS No. | 58880-19-6 |
| Molecular Formula | C17H22N2O3 |
| Molecular Weight | 302.37 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 10 mM in ethanol and to 50 mM in DMSO |
| Source | Synthetic |
| Appearance | Beige Solid |
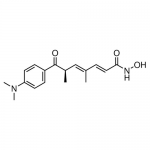
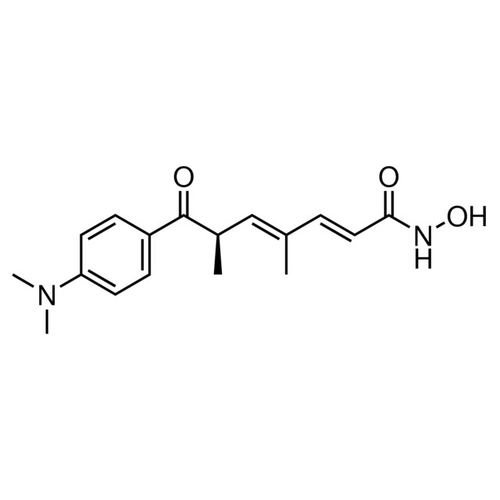
| SMILES | C[C@H](/C=C(C)/C=C/C(=O)NO)C(=O)C1=CC=C(C=C1)N(C)C |
| InChI | InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+ |
| InChIKey | RTKIYFITIVXBLE-WKWSCTOISA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection Risk Phrases: R20/21/22 - Harmful by inhilation, in contact with skin and if swallowed R68 - Possible risk of irreversible effects Hazard Phrases: H302-H312-H315-H317-H319-H332-H335 Precautionary Phrases: P261-P280-P305 + P351 + P338 |
| Cite This Product | Trichostatin A (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-254) |
Biological Description
| Alternative Names | (2E,4E,6R)-7-[4-(Dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide |
| Research Areas | Apoptosis, Cancer, Cancer Growth Inhibitors, HDAC Inhibitors |
| PubChem ID | 444732 |
| Scientific Background | Trichostatin A (TSA) is a potent and selective inhibitor of class I and II histone deacetylases (HDACs). By preventing histone deacetylation, TSA promotes chromatin relaxation and transcriptional activation. In neuroscience, TSA is used to study epigenetic regulation of gene expression, synaptic plasticity, and memory formation. It has shown promise in models of neurodegenerative diseases by enhancing neuroprotective gene expression and reducing neuroinflammation. |
| References |
1. Vanhaecke T., Papeleu P., Elaut G., Rogiers V. (2004) Curr Med Chem. 11(12): 1629-1643. 2. Yoshida, et al. (1990) J Biol Chem. 165: 17174. 3. Drummond D.C., et al. (2005) Annu Rev Pharmacol Toxicol. 45: 495-528. |

Reviews
There are no reviews yet.