The Role of Rab1a in Cancer and Neurodegeneration
Rab1a, a small GTPase, is best known for its role in mediating vesicular trafficking from the endoplasmic reticulum (ER) to the Golgi apparatus. However, Rab1a is also involved in many other essential cellular processes, and has recently been linked to a range of human diseases including cancer, Parkinson’s disease, and cardiomyopathy. By understanding how Rab1a may be exploited as a drug target, researchers aim to improve patient survival rates through more effective therapeutic intervention.
What is the Rab family?
The Rab family is the largest branch of the Ras superfamily, comprising more than 60 family members in humans1. To put this into context, 167 members of the Ras superfamily have been identified in humans to date and are divided into five main subfamilies – Ras, Rab, Rho, Arf and Ran2. Like all Ras superfamily proteins, Rabs are small GTPases that cycle between a membrane-associated (GTP-bound) active form and a cytosolic (GDP-bound) inactive conformation to carry out their designated role3. Each Rab protein is associated with a specific organelle and its related cargo vesicles, where it functions to identify the cargo material and ensure it is transported correctly1.
What is Rab1a?
Rab1a is one of two isoforms of Rab1, sharing 92% homology with Rab1b4. It is localized to the ER and Golgi apparatus, where it mediates ER-to-Golgi trafficking, and it is also involved in cell signaling, cell migration, nutrient sensing, and the regulation of autophagy1,5. Both Rab1a and Rab1b are evolutionarily conserved, being found in found in 158 and 89 different organisms, respectively6. Each consists of a short N-terminal sequence, followed by a “G box” that is essential for guanine nucleotide binding and GTP hydrolysis, and a C-terminal “CC motif” containing two cysteine residues, for membrane targeting via geranylgeranyl modification6. While Rab1a is 205 amino acids in length, Rab1b comprises 201 amino acids.
How is Rab1a involved in cancer?
Aberrant expression of Rab1a has been reported in many different types of cancer, including hepatocellular carcinoma (HCC), colorectal cancer (CRC), prostate cancer, glioma, lung cancer, and tongue squamous carcinoma5,6. In HCC and CRC, tumors expressing high levels of Rab1a have been shown to exhibit hyperactive mTORC1 signaling, which in turn promotes tumor growth, invasion, and poor prognosis6,7,8. Yet, while over-expression of Rab1a has been observed during histopathological analysis of various lung cancer tissues, Rab1a knockdown in lung cancer cell lines has proven to have little effect on mTORC1 signaling or cell growth5. This indicates that Rab1a may behave differently in the pathogenesis of different cancers.
How does Rab1a interact with alpha synuclein?
The accumulation of alpha synuclein in neurons is a classic hallmark of Parkinson’s disease (PD). In a study designed to reveal the effects of inducing alpha synuclein expression in yeast, a block in ER-to-Golgi vesicular trafficking was one of the earliest defects to be observed. This was accompanied by the observation that Ypt1p (the yeast homolog of Rab1a) associated with cytoplasmic alpha synuclein inclusions and led to the discovery that elevated Rab1 expression could protect against alpha synuclein-induced dopaminergic neuron loss in animal models of PD9. A subsequent study has demonstrated that alpha synuclein compromises autophagy by inhibiting Rab1a and that this can be overcome by Rab1a over-expression10.
Supporting the study of Rab1a
We offer a broad range of products for studying Rab1a, inclduing two mouse monoclonal RAB1A Antibodies, clone 4G10 and clone 7H4 Both are validated for Western blotting and immunoprecipitation and are available as a various conjugates. Both antibodies have been tested against lysates derived from Rab1a knockout cell lines for results you can trust.
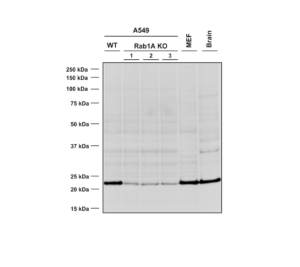
Western Blot analysis using Mouse Anti-RAB1A Monoclonal Antibody, Clone 4G10 (catalog# SMC-613). Lane 1 – WT A549 cells, lanes 2-4 – Rab1A KO A549 clones, lane 5 – MEF cells, lane 6 – mouse brain cells.
REFERENCES
- Role of Rab GTPases in Membrane Traffic and Cell Physiology, Hutagalung AH and Novick PJ, Physiol Rev. 2011 Jan; 91(1): 119–149.
- The Ras Superfamily of Small GTPases in Non-neoplastic Cerebral Diseases, Qu L et al, Front Mol Neurosci. 2019 May 21;12:121.
- Rab proteins and the secretory pathway: the case of Rab18 in neuroendocrine cells, Vázquez-Martínez R and Malagón MM, Front Endocrinol (Lausanne). 2011 Jan 17;2:1.
- Proteomic analysis of endocytic vesicles: Rab1a regulates motility of early endocytic vesicles, Mukhopadhyay A et al, Journal of Cell Science 2011 124: 765-775.
- Expression of Rab1A is upregulated in human lung cancer and associated with tumor size and T stage, Wang X et al, Aging (Albany NY). 2016 Nov 29;8(11):2790-2798.
- Rab1 in Cell Signaling, Cancer and Other Diseases, Yang X et al, Oncogene. 2016 Nov 3;35(44):5699-5704.
- Rab1A Is an mTORC1 Activator and a Colorectal Oncogene, Thomas JD et al, Cancer Cell. 2014 Nov 10;26(5):754-69.
- Rab1A promotes proliferation and migration abilities via regulation of the HER2/AKT-independent mTOR/S6K1 pathway in colorectal cancer, Cheng Z et al, Oncol Rep. 2019 May;41(5):2717-2728.
- Alpha-Synuclein Blocks ER-Golgi Traffic and Rab1 Rescues Neuron Loss in Parkinson’s Models, Cooper AA et al, Science. 2006 Jul 21;313(5785):324-8.
- Alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease, Winslow AR et al, J Cell Biol. 2010 Sep 20;190(6):1023-37.


Leave a Reply