Novel Chemiluminescence Assay for Detecting Misfolded Proteins in Neurodegenerative Diseases
Neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis (ALS) are defined by the progressive accumulation of misfolded protein aggregates in the brain. These abnormal protein conformations disrupt normal cellular function triggering neuroinflammation and cell death. These normally soluble proteins aggregate into β-sheet–rich fibrils which further seed toxic cascades throughout the brain. This type of neurological degeneration, known as proteinopathy, is increasingly common in older adults. However, early detection and monitoring of misfolded proteins have proved challenging. Current tools struggle with low sensitivity, high background fluorescence, and poor performance in living tissue. Most notable among these tools is the fluorescent dye thioflavin T (ThT), a dye used to detect β-sheet structures.
To address this issue, researchers at the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital/Harvard Medical School, have developed a new chemiluminescent probe. This probe is engineered for both generic detection of β-sheet structures and precise targeting of individual misfolded proteins. In a recent preprint published in bioRxiv, Zhu et al. test their probe ADLumin-1 against thioflavin T on a variety of peptides and proteins associated with proteinopathy. They call these proteins “misfoldon”, as they share a common β-sheet structure associated with this type of misfolded protein. Additionally, they frequently contain the amino acids alanine, valine, isoleucine, leucine and phenylalanine. These amino acids can form hydrophobic pockets within the β-sheet.
ADLumin-1 binds specifically to β-sheet structures with superior results compared to ThT
Upon observing an increase in chemiluminescent signal from ADLumin-1 in the presence of amyloid beta fibrils, Zhu et al. determined that ADLumin-1 was binding to the hydrophobic pockets inside the β-sheet fibrils, along their long axis. This binding is similar to that of thioflavin T. Based on these findings, Zhu et al. hypothesized that ADLumin-1 could act as a generic probe for β-sheet structures. To test this, they used model peptides PA-K2 and PA-E2 – which form β-sheet fibrils (rich in Val/Ala). Additionally, they used peptides PA-K and PA-E, which assemble into fibrils without extensive β-sheets.
They researchers demonstrated that ADLumin-1’s chemiluminescence increased 4,168-fold with PA-K2 against background. This was compared to around 248-fold with PA-K, an intensity ratio of 16.8. In comparison to ThT, incubation with PS-K2 ADLumin-1 delivered a 127.7-fold higher signal-to-noise (SNR) ratio than thioflavin T. Similar results were obtained with the PA-E/PA-E2 peptides. Having demonstrated ADLumen-1’s ability to specifically and stably bind to β-sheet structures with higher sensitivity than ThT, the researchers decided to test it against a selection of disease-relevant misfoldons.
Upon mixing with StressMarq’s Tau (K18) P301L Mutant Pre-formed Fibrils (catalog# SPR-330), alpha synuclein, amyloid beta, and TDP-43 fibrils, strong chemiluminescent signals were detected with a superior signal-to-noise ratio (SNR) versus ThT. Compared to ThT, ADLumin-1 improved SNR by 83-fold for alpha synuclein, 75-fold for amyloid beta, 15-fold for tau and 11-fold for TDP-43. Further, ADLumin-1 could also differentiate between monomers and fibrils and was able to detect oligomers. The variable signal decay half-life times between misfoldons indicated that ADLumin-1 could potentially be used to distinguish between them. A molecular docking experiment confirmed ADLumin-1 docks along the long axis of β-sheet fibrils, engaging hydrophobic tunnels formed by Ala, Val, Ile, and Phe. This docking explains its broad reactivity.
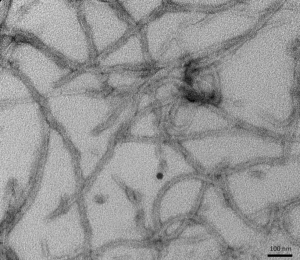
Figure 1. [Figure from StressMarq website] Transmission electron microscopy (TEM) of Tau (K18), P301L mutant Pre-formed fibrils (PFFs) (catalog# SPR-330)
ADLumin-1 can be used for in vivo imaging of Tau and TDP-43
Tau is a key protein associated with Alzheimer’s disease (AD) where it aggregates and forms tangles in AD patient brains. ADLumin-1 was able to colocalize with anti-tau antibodies in stained AD patient brain sections. In wild-type (WT) and mutant tau P301 mice, after injection of ADLumin-1, chemiluminescence was recorded in an animal imaging system over different timepoints. Large differences in chemiluminescence were observed between the mutant and WT mice. Both tau studies demonstrate effective detection of tau tangles in vivo.
In amyotrophic lateral sclerosis (ALS), TDP-43 aggregates in the motor neurons of the brain and spinal cord. In a similar experiment on wild-type and A315T transgenic mice (an ALS model), intravenous probe delivery produced a higher signal in A315T mice compared to WT. On A315T mouse sections, inclusions were detected by ADLumin-1 and confirmed by antibody colocalization.
In vivo imaging of alpha synuclein can be enhanced by ChRET
In Parkinson’s disease (PD), alpha synuclein aggregation plays a central role forming toxic oligomers and fibrils. As such, reliable detection of alpha synuclein both in vitro and in vivo would be invaluable for PD research. Zhu et al. were able to detect alpha synuclein to a femtomolar level of sensitivity, which surpassed that of ThT. Using a combination of protein misfolding cyclic amplification (PMCA) and seeding, they were able to detect alpha synuclein in cerebrospinal fluid (CSF). This detection opens the possibility for its use for in vitro diagnostics. Importantly, ADLumin-1 was also capable of detecting alpha synuclein in PD disease patient brain tissues. This is crucial, as it could lend itself to use in postmortem analysis of brain samples.
In an in vivo study performed by the researchers, mice intracranially injected with alpha synuclein aggregates were labelled with ADLumin-1. They then used three-dimensional diffuse luminescent imaging tomography (DLIT). This technique revealed localized chemiluminescence only at seeded sites, which confirmed selective deep-brain detection of alpha synuclein. Transgenic A53T mice overexpressing human alpha synuclein showed 2.1-fold higher brain signal than wild-types after intraperitoneal ADLumin-1. In a longitudinal analysis from 4 to 12 months, signal ratios between A53T and WT mice rose to 2.6-fold, mirroring disease progression. They were then able to verify the alpha synuclein accumulation by ex vivo histology staining.
Having confirmed that ADLumin-1 can track alpha synuclein spread in vivo, the scientists developed a method to both increase the signal and selectively image alpha synuclein. They used a bio-orthogonal chemiluminescence resonance energy transfer (ChRET) technique, which pairs ADLumin-1, a chemiluminescent donor, with a fluorophore acceptor. This acceptor binds specifically to alpha synuclein (CRANAD-14). The use of this method enabled precise alpha synuclein imaging in living transgenic A53T mouse brain, differentiating between the misfolded amyloid beta in an Alzheimer’s transgenic mouse model (5xFAD).
Summary
Achieving signal-to-noise ratios up to 128-fold greater than thioflavin T, ADLumin-1 is a promising new reagent for the detection of misfolded proteins associated with neurodegeneration. Proteins such as amyloid beta, tau, alpha synuclein, and TDP-43 pathologically aggregate into fibrils, adopting a hydrophobic β-sheet structure to which ADLumin-1 binds. Moreover, this chemiluminescent probe has the advantage of greater sensitivity, no autofluorescence, and deep tissue penetration. It can detect alpha synuclein in cerebrospinal fluid at femtomolar sensitivity and non-invasively visualize aggregates of tau, alpha synuclein, and TDP-43 in live transgenic mouse disease models, a feat unachievable with thioflavin T.
In addition, when the researchers combined ADLumin-1 with their own fluorescent probe CRANAD-14 in a bio-orthogonal ChRET assay, they were able to precisely image alpha synuclein in living mouse brain and differentiate between alpha synuclein and amyloid beta. This highly sensitive, in vivo technique could prove to be a valuable tool for research and monitoring of multiple proteinopathies.
Related StressMarq products
StressMarq offers a full suite of neuroscience research reagents. Complement your studies with rigorously validated monomers, oligomers and pre-formed fibrils for tau, alpha synuclein, and amyloid beta proteins. To replicate the pathological aggregation seen in Alzheimer’s disease, StressMarq’s Tau dGAE (297-391) AD-mimic Pre-formed Fibrils (catalog# SPR-502) are a reliable tool for modelling tauopathy and advancing therapeutic discovery.
References
- Highly sensitive chemiluminescence imaging of misfolded proteins in neurodegenerative models. Zhu, B. et al., Proc. Natl. Acad. Sci. U.S.A.
- Detection of misfolded proteins in neurodegenerative disease models with a highly sensitive chemiluminescence. Zhu, B. et al., bioRxiv, (preprint)
Topics covered in this post: Tau, neurodegeneration, proteinopathy, amyloid beta, alpha synuclein, TDP-43, Parkinson’s disease, Alzheimer’s disease and amyotrophic lateral sclerosis (ALS).


Leave a Reply