Artificial miRNA Strategies for SNCA Knockdown in Alpha Synucleinopathies
Parkinson’s disease (PD) is a neurodegenerative disease characterized by tremors, rigidity, and bradykinesia, which is a slowing of the movements of the body. PD has been classified as a synucleinopathy, a family of disorders which involve the accumulation of alpha synuclein, a presynaptic regulatory protein, in the brain. Synucleinopathies and related proteinopathies have become increasingly common in older adults, and as such there has been an increased effort to understand the mechanisms of action and elucidate treatments for these diseases.
Potential treatments for Parkinson’s disease have mostly been aimed at targeting alpha synuclein itself, though clinical research is still in the early stages. Alpha synuclein is coded by the SNCA gene, and both missense mutations and single nucleotide polymorphisms (SNPs) in this gene have been associated with increased risk of developing Parkinson’s disease. As such, researchers at Sanofi’s Genomic Medicine Unit set out to examine the efficacy of treatments using adeno-associated viral (AAV) mediated SNCA reduction via artificial microRNAs (amiRNAs). Using a pre-formed fibril (PFF) model of alpha synucleinopathy, Elmer et al. evaluated the therapeutic potential of amiRNAs in targeting SNCA mRNA levels, optimizing these amiRNAs both in vivo and in vitro for clinical translation.
amiRNA treatment results in 50% reduction of target mRNA
The researchers began by running a dose-ranging study with the aim of determining the dose of AAV-mediated amiRNA needed for a 50% reduction in Snca (mouse) expression. They began by assessing target reductions in wild-type mice, and followed that by evaluating mice subjected to bilateral intrastriatal injections of StressMarq’s Mouse Recombinant Alpha Synuclein PFFs (catalog# SPR-324), which induced alpha synuclein pathology.
This study used AAV PhPeB vectors which are a proven brain-penetrant capsid in rodents. Wild-type mice were treated with either amiRNA that expressed homology to mouse Snca (amiRmSYN) at three dosage levels, or with a control amiRNA (amiRCTL). The highest dose level of amiRmSYN produced the target of up to 50% reduction in Snca expression and was therefore used in the subsequent mouse models of Parkinson’s pathology.
StressMarq’s Mouse Recombinant Alpha Synuclein Monomers (catalog# SPR-323) were used as a control, and neuropathological efficacy readouts were utilized for study outcomes, as minimal motor deficits were observed after both 1.5 and 3 months. Mice were initially treated with AAV vectors which encoded either amiRmSYN or amiRCTL, then injected with alpha synuclein monomers or PFFs one month later. Brain tissue was collected three months post-injection. Similar to the results in wild-type mice, treatment with amiRmSYN resulted in an approximate 50% reduction of target mRNA. Additionally, alpha synuclein knockdown with amiRmSYN significantly reduced the induction of pSer129 pathology. Subjects treated with monomers or PFFs in combination with amiRCTL did not have altered Snca expression.
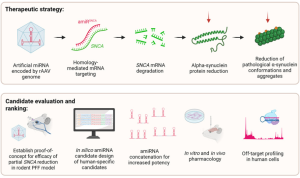
Figure 1. Graphical abstract from Elmer et al.
Enhanced potency for human-specific amiRNA candidates
After validating the proof-of-concept for AAV-mediated amiRNA treatment, the research group turned their attention to optimizing these amiRNAs for potential therapeutic targeting in humans. Using in silico down selection, they found 14 candidate amiRNA sequences. These sequences were evaluated using enzyme linked immunosorbent assay (ELISA) and were all found to diminish alpha synuclein protein levels.
The researchers then analyzed five of the previously identified amiRNAs in a dose-response experiment using HeLa cells. Of these five, three amiRNAs outperformed the previous amiRmSYN amiRNA used in the PFF mouse model, showing promising early results for potential efficacy in human PD models.
Following these results, Elmer et al. aimed to increase SNCA knockdown through enhancing amiRNAs via concatenation. They identified four amiRNA parental guide strands, and for each of these strands they developed concatenated variants expressing either two (“2X”) or three (“3X”) copies of the amiRNA hairpin. miRNAs often occur in a naturally concatenated form with several hairpins, and artificial concatenation has been previously established as an effective method to increase target repression.
Additionally, the research group sought to evaluate whether varying the distance between these amiRNA hairpins would affect the potency. They did so by inserting spacer sequences in both the 2X and 3X variants and comparing their potency against a non-concatenated single copy (“1X”). SNCA knockdown was measured via transfecting the amiRNA plasmids into HeLa cells, and they found that nearly all the 2X and 3X variants outperformed the 1X design. This study also found that concatenation had nominal effects on processing of the guide strand.
In vivo SNCA reduction and RISC interaction mapping of amiRNA variants
The next step for this research was to investigate the efficacy of the concatenated amiRNAs against human alpha synuclein in an in vivo model. This was accomplished using a transgenic mouse line developed by the Michael J Fox Foundation, which crucially has the SNCA gene integrated into the genome, making it susceptible to mRNA targeting. The amiRNAs were injected intrastriatally using an AAV capsid, and the striatal tissue was collected six weeks after injection. In line with previous results, they found that increased expression of amiRNA in the 3X variants created a significant decrease in Snca levels.
Finally, this study examined the downstream effects of SNCA knockdown using a combinatory approach of differentially based genes (DEG)-based analysis, cross-linking, immunoprecipitation, and RNA sequencing. This combinatory method had been previously used to quantify miRNA:mRNA interactions, and the researchers were able to identify one parent amiRNA with off-targets and three parent strands that had no identifiable off-targets.
Summary
Elmer et al. tested the efficacy of AAV-mediated artificial miRNA in blocking the spread of pathogenic alpha synuclein protein. They did so in a variety of settings, using in vivo and in vitro models, and targeting both rodent and human SNCA knockdown to diminish Snca expression, and therefore interrupt the spread of pathology in Parkinson’s disease models. Their findings introduce novel potential therapeutic mechanisms for combatting Parkinson’s disease and establish 50% SNCA suppression as a target for future amiRNA research.
Related StressMarq products
Continued research into therapeutic targets for neurodegenerative diseases is crucial. To support this cutting-edge research, StressMarq Biosciences manufactures a wide range of specialized proteins for modelling neurodegenerative diseases such as Alzheimer’s and Parkinson’s. These preparations include monomeric, oligomeric and fibrillar tau, alpha synuclein and amyloid beta proteins for disease modelling and pre-clinical drug discovery. To learn more about how StressMarq products are being used to drive advances in neurodegeneration research, visit our product citations page.
References
Artificial miRNA mediated reduction of SNCA for the treatment of α-synucleinopathies. Elmer, B. et al., Mol Ther. 2025


Leave a Reply