A Neuroprotective Compound in Traditional Chinese Medicine
Alzheimer’s disease (AD) is the leading cause of dementia worldwide, with vascular, frontotemporal and Lewy body (LB) dementias following closely behind. It is characterized by the abnormal accumulation of neuritic plaques and neurofibrillary tangles (NFTs), which consist of aggregated amyloid beta (Aβ) and hyperphosphorylated tau, respectively. These aggregates trigger inflammation, disrupt synaptic function, induce neuronal loss, and ultimately result in cognitive decline and behavioral changes.
Current treatments primarily focus on the alleviation of symptoms rather than targeting the underlying molecular mechanisms that drive the onset and progression of AD. Approved therapeutic interventions such as acetylcholinesterase (AChE) inhibitors help slow symptom progression, but are unable to inhibit the processes that cause them.
In the search for disease-modifying therapies, researchers are exploring pharmacological compounds derived from traditional Chinese medicine. With a history spanning over 2,200 years, this ancient practice has been widely used to support various health conditions, including immune disorders, cardiovascular diseases, cancer, and COVID-19. Academic studies are now investigating well-known compounds involved in traditional Chinese medicine (TCM) to determine if bioactive compounds can address the pathophysiological processes driving neurodegeneration.
Alternative medicine
One widely used component of Chinese medicine is the plant Penthorum chinense Pursh (PCP). PCP is a perennial herb, meaning it survives multiple growing seasons without needing to be replanted. It thrives in forests, meadows, and wetlands across East Asia, particularly in China, Japan, and South Korea. In these regions, PCP is valued for its medicinal properties and is commonly used to treat various health conditions, ranging from pain relief to liver ailments.
Modern medical research is working to extract bioactive compounds from well-known traditional sources, in order to identify their pharmacological properties and molecular targets. In the search for new pharmacological agents to slow the progression of Alzheimer’s disease, a study by Yu et al., published in Phytotherapy Research, isolated a bioactive compound called thonningianin A (TA) from PCP. In experimental studies, TA demonstrated the ability to bind to the pathogenic amyloid beta and tau proteins associated with Alzheimer’s disease, reducing their cytotoxicity in neuronal cells. The discovery of Thonningianin A’s neuroprotective capabilities underscores the untapped potential of Eastern medicinal remedies within the context of neurodegenerative disease research.
Asssessing amyloid beta fibrillization
To initiate the study, researchers from Southwest Medical University in Sichuan, China, examined whether components of traditionally used PCP plant possessed neuroprotective properties. To evaluate this, they assessed the impact of Penthorum chinense Pursh on the fibrillization of amyloid beta. A specific fragment of amyloid beta, known as amyloid beta 1-42, plays a crucial role in Alzheimer’s disease. This fragment is produced through the sequential cleavage of a membrane-bound amyloid precursor protein (APP) by β- and γ-secretases. In AD, Aβ 1-42 exhibits a heightened tendency to form amyloid fibrils, which subsequently accumulate into plaques and contribute to neurotoxicity. Neuroprotective pharmacological agents that inhibit amyloid beta fibrillization—or fibril formation—may help slow the progression of neurodegenerative diseases.
Ethanol-based solvent extraction is one of the safest and most common methods for isolating soluble components from plants. Using this approach, the researchers first tested whether the total fraction of the whole PCP plant could inhibit amyloid beta fibrillization. The fluorescent dye thioflavin selectively binds to pathogenic amyloid beta fibrils, and was used to detect changes in fibrillization. Indeed, the total fraction of PCP demonstrated a dose-dependent inhibitory effect on the fibrillization of Aβ 1-42.
Next, different parts of the PCP plant—leaf, flower, and stem—were separated, and their soluble components were extracted using the same ethanol-based method. Among these, the flowers of PCP, which were found to contain thonningianin A as the main component, also exhibited the ability to inhibit Aβ 1-42 fibrillization. This finding led researchers to further investigate the role of TA in modulating the molecular mechanisms underlying Alzheimer’s disease.
Analyzing molecular interactions
To investigate the molecular behaviour of thonningianin A, investigators employed bio-layer interferometry (BLI) to analyze its potential interactions with amyloid beta and tau. BLI is an optical biosensing technique that enables real-time analysis of biomolecular interactions. As it does not require the use of a fluorescent label, BLI eliminates potential label interference while providing accurate real-time data, including binding affinity and kinetics.
The experimental setup for BLI was identical for both the amyloid beta 1-42 peptide and tau. For the latter, researchers focused on the P301L mutation, a well-documented pathogenic variant in neurodegenerative disease that leads to increased tau phosphorylation, aggregation, and propagation. In order to closely analyze this interaction, the BLI sensor was prepared by immobilizing biotinylated StressMarq Tau (K18) P301L Mutant Pre-formed Fibrils (catalog #SPR-330) onto the sensor tip. The sensor tip was then dipped into a solution containing the TA analyte to assess interaction and potential binding. The resulting association and dissociation curves, alongside steady-state analysis confirmed that TA was capable of binding to both tau and Aβ 1-42.
Amyloid beta and tau pathology
Next, the study investigated whether TA could mitigate the cytotoxic effects caused by amyloid beta and tau. To mimic the toxic impact of these proteins, a PC12 neuronal model was generated using transient transfection. Neuronal damage was then assessed using MTT cytotoxicity assays and flow cytometry to analyze cell apoptosis. The results demonstrated that treatment with TA significantly alleviated amyloid beta- and tau-induced cytotoxicity, leading to a dose-dependent reduction in cell apoptosis and decreased cell death.
With the hope of further exploring the neuroprotective effects of TA in a more complex and dynamic biological system, the 3xTg-AD mouse model was subsequently employed. This transgenic model carries three mutations associated with familial Alzheimer’s disease (APP Swedish, MAPT, and PSEN1 M146V), leading to progressive amyloid beta deposition and hyperphosphorylated tau accumulation. These pathological changes facilitate the formation of plaques and tangles, which in turn contribute to cognitive impairments, particularly in spatial learning and memory.
The findings revealed that TA treatment reduced molecular markers of AD, as evidenced by decreased amyloid beta deposits and phosphorylated tau levels observed in immunohistochemical staining and western blot analyses. Moreover, TA administration alleviated Alzheimer’s disease-like cognitive impairments and inhibited neuronal cell death, highlighting its potential as a therapeutic agent for neurodegenerative diseases.
Measuring molecular interactions with StressMarq’s neurodegenerative protein constructs
This study highlights the neuroprotective effects of thonningianin A, a compound derived from a staple medicinal herb involved in traditional Chinese medicine. Using biolayer interferometry, researchers examined the molecular interactions between key pathogenic proteins. Notably, TA exhibited specific binding to StressMarq’s Tau (K18) P301L Mutant Pre-formed Fibrils (catalog #SPR-330), which contain the pathogenic P301L mutation commonly associated with neurodegenerative disease pathology. Additionally, TA demonstrated a strong binding affinity for amyloid beta 1-42. These findings underscore the valuable contributions of traditional Chinese medicine and highlight its potential as an untapped source for developing pharmacological agents to combat neurodegenerative disease.
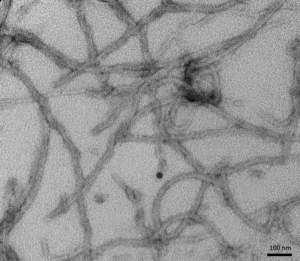
[Image from: StressMarq website] TEM imaging of StressMarq’s Tau (K18) P301L Mutant PFFs (catalog# SPR-330).
Summary
Current treatments for Alzheimer’s disease primarily focus on alleviating symptoms rather than targeting the underlying molecular mechanisms. In the search for disease-modifying drugs, researchers are exploring traditional Chinese medicinal plants for potential bioactive therapeutic compounds. Recent findings by Yu et al. highlight the neuroprotective effects of thonningianin A, a compound derived from the Penthorum chinense Pursh plant. TA binds to amyloid beta and tau with high affinity, reducing their cytotoxicity in cells, enhancing cognitive function, and alleviating Alzheimer’s pathology. These results suggest that TA could serve as a promising bioactive compound in the development of nutraceutical and pharmacological agents to treat Alzheimer’s disease.
Related StressMarq Products
StressMarq manufactures a diverse range of tau protein constructs that include disease-relevant isoforms and mutations associated with Alzheimer’s disease. The study of tau ΔK280 mutations — which are strongly linked to neurodegenerative disease pathology — is facilitated by StressMarq’s Tau (K18) Delta K280 Mutant Pre-formed Fibrils (catalog #SPR-477). Visit our website for more information, including the latest scientific publications using our specialized tau, alpha synuclein and amyloid beta proteins for neurodegenerative disease research.
References
- Thonningianin A from Penthorum chinense Pursh as a targeted inhibitor of Alzheimer’s disease-related β-amyloid and tau proteins. Yu, L. et al., Phytother Res. 2025.


Leave a Reply