Alzheimer’s Disease and Lysosome Dysfunction
Alzheimer’s disease (AD) is the most common cause of dementia, characterized by the intracellular accumulation of tau proteins forming neurofibrillary tangles, and the extracellular aggregation of amyloid beta (Aβ) into senile plaques. Microglia, phagocytic immune cells found in the brain, are responsible for clearing these pathogenic proteins. Consequently, microglia dysfunction and mutations affecting genes highly expressed in microglia are of particular interest to Alzheimer’s disease researchers. In Alzheimer’s disease pathology microglia migrate and cluster around amyloid beta plaques but are unable to remove them. A potential explanation for this dysfunction is impairment of lysosome activity, which could affect the phagocytic ability of microglia.
Researchers at the University of Washington, USA had previously shown that the loss of an intracellular sorting protein in their human microglia cell model could result in enlarged lysosomes. This protein is known as SORLA (sortilin-related receptor protein) and is encoded by SORL1 and highly expressed by microglia. Mutations in the SORL1 (sortilin-related receptor-1) gene are associated with AD. In their most recent study, Mishra et al. investigated its function as a receptor and regulator of lysosomal trafficking. Their aim was to understand how decreased levels of SORLA expression, as observed in AD patients, impact lysosome function within microglia and how this could relate to Alzheimer’s. Using human induced pluripotent stem cells, Mishra et al generated wild type and SORL1 knock out microglia like cells (hMGLs) to investigate the effects of SORLA deficiency on lysosomal function.
Reduced lysosomal degradation
Treatment of wild type (WT) and knock out (KO) hMGLs with DQ-BSA, a substance which only fluoresces when degraded in lysosomes, followed by flow cytometry to measure intracellular fluorescence intensity, revealed reduced lysosomal degradation in SORL1 KO hMGLs compared to the WT control. This was originally suspected to be due to reduced lysosomal enzyme activity. As overall lysosomal protein expression was found to be unaffected, researchers looked for an alternative explanation for this reduction by examining activity in cells lacking SORLA.
Movement of lysosomal hydrolases from the trans Golgi network (TGN) to lysosomes can occur via a pathway which is regulated by the cation independent mannose-6-phosphate receptor (CIMPR) and the multi-protein complex retromer. To determine if loss of SORLA interfereswith this function, the researchers used antibodies against the lysosomal enzymes cathepsin B, cathepsin D and HEXB to measure colocalization with markers for the TGN and late endosome/lysosome in an immunocytochemical analysis. SORL1 KO hMGLs showed increased colocalization with the TGN marker, TGN38 and decreased colocalization with the lysosome marker, LAMP1, compared to WT. These results demonstrated mis-trafficking of lysosomal enzymes from the TGN to the lysosome. Next, the researchers looked at the trafficking protein CIMPR to determine how the loss of SORLA is causing these enzymes to remain in the TGN. Using a colocalization experiment and western blot analysis, they found lower expression of CIMPR, and reduced colocalization with LAMP1 in KO hMGLs. Taken together, the data suggested that the CIMPR-dependent trafficking pathway is impaired in SORL1 knock-out hMGLs.
Phagocytosis and lysosomal stress
The experiment then assessed whether phagocytosed substrates in lysosomes accumulate due to reduced lysosomal catabolism in SORL1 knock-out hMGLs. Accumulation of fluorophore-labeled amyloid beta 1-42 fibrils (fAb) and dye-labeled synaptosomes were measured and found to increase in cells lacking SORLA. On inhibition of catabolism by chloroquine, both WT and KO demonstrated increased accumulation of these substrates. To check if increased phagocytotic uptake is contributing to the accumulation in KO cells, they were again incubated with fAb, the dye-labeled synaptosomes, along with StressMarq’s Amyloid Beta 1-42 Oligomers (catalog# SPR-488). Interestingly, uptake was increased for the synaptosomes and fibrils but not for the oligomers in the KO cells, suggesting increased phagocytosis may be substrate specific. Phagocytic cell surface receptors TREM2 and P2Y6 showed increased cell surface localization on SORL1 KO hMGLs, suggesting SORLA maintains optimum phagocytic receptor levels on the cell surface. Therefore, it was concluded that an increased uptake and decreased degradation of phagocytosed substrate is a key factor behind lysosomal dysfunction and stress in SORL1 KO hMGLs.
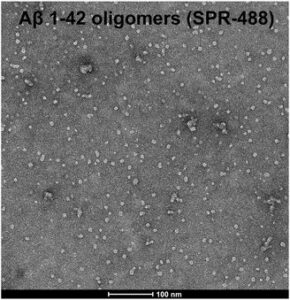
Figure 1. [Image from: StressMarq website] Transmission electron microscopy (TEM) imaging of Amyloid Beta 1-42 Oligomers (catalog# SPR-488).
Summary
This study sought to understand how mutations in SORL1 increase the risk of Alzheimer’s disease. Using an in vitro microglia-like cell model, researchers observed mis-trafficking of lysosomal enzymes leading to impaired lysosomal degradation. Phagocytosis was increased in a substrate specific manner, with amyloid beta and synaptosomes accumulating in lysosomes. The LE alternative pathway was also diminished leading to reduced levels of extracellular lysosomal enzymes and cytokines involved in inflammatory responses. These combined effects could lead to lysosomal stress, accumulation of toxic protein within cells, and a reduction in the pro-inflammatory responses that deal with AD pathology. The findings described in this study highlight targeting microglia and their role in regulating lysosomal pathways as a potential therapeutic strategy for the treatment of Alzheimer’s disease.
Related StressMarq products
StressMarq Biosciences is world leading manufacturer of a range of specialized proteins for modelling neurodegenerative disease such as Alzheimer’s and Parkinson’s disease. These preparations include monomeric, oligomeric and fibrillar tau, alpha synuclein and amyloid beta proteins for disease modelling and pre-clinical drug discovery. To learn more about how StressMarq products are being used to drive advances in neurodegeneration research, visit our Product Citations page.
References
The Alzheimer’s disease gene SORL1 regulates lysosome function in human microglia. Mishra, S. et al. Glia. 2025.

Leave a Reply