How Disease Models Advance Neurodegenerative Disease Research
Neurodegenerative diseases are age-dependent disorders that result in the degeneration of the nervous system, most commonly the neurons in the brain. These diseases are incurable, and though the field of neurodegeneration research is growing rapidly, the molecular mechanisms underpinning neurodegeneration remain largely unknown. This is due in part to the complexity of neurodegenerative diseases, as each disease (e.g. Alzheimer’s, Parkinson’s, ALS) has heterogenous pathology that can vary widely between patients.
As researchers have sought to better understand and develop treatments for these neurodegenerative diseases, disease modelling has emerged as a critical tool. There are three broad categories of disease modelling: in vivo, in vitro, and computational, and each of them have their part to play in the advancement of neurodegenerative disease research.
In vivo modelling
In vivo modelling is the practice of inciting and observing disease pathology within living organisms. For neurodegenerative disease research, researchers often use rodent models. Although, researchers have accomplished modelling within yeast, zebrafish, fruit flies, and other organisms. Notably, the widespread adoption of transgenic models—models where DNA from other species is successfully implanted into the host species—has been crucial for studying neurodegeneration. This method allows researchers to more closely observe mechanisms of actions for pathology-inducing aggregating proteins such as alpha synuclein, tau, and amyloid beta.
A recent example of such research is the paper “Artificial miRNA-mediated reduction of SNCA for the treatment of α-synucleinopathies” by Elmer et al., which used transgenic mise to study the efficacy of RNA-mediated therapeutics to reduce alpha synuclein expression and pathology.
However, in vivo models are not without their challenges. Choosing a model species can be complicated and costly, and there is no guarantee that the disease will behave the same way in the model species as it would in humans. As such, in vitro and computational models can help fill the gaps.
In vitro modelling
By contrast, in vitro models are done in lab settings, outside of living organisms. They address some of the concerns with animal models: they are typically less expensive, highly customizable, and can more accurately reflect human cellular pathways. In a Nature Neuroscience publication, Choi et al. used StressMarq’s Amyloid Beta 1-42 Oligomers (catalog# SPR-488) to examine the role of Sox9 expression in amyloid beta phagocytosis in Alzheimer’s disease. Several types of in vitro models are widely used in neurodegenerative research, including those listed below:
Organoids: These models are 3D cell cultures that can preserve the cellular interactions found in human organs. Specifically, human induced pluripotent stem cells (hiPSCs) have emerged as a key tool for neurological modelling. hiPSCs can differentiate into many cell types, self-renew, and are obtained from somatic cells in humans. These characteristics allow for a more accurate disease model.
Immortalized cell lines: These are cell lines that are either cancerous or have been altered to reproduce indefinitely. They are widely used within disease modelling and drug development due to their rapid growth, low cost, and reproducibility. Additionally, they have been combined with astrocytes and neurons to model the blood–brain barrier in drug development.
Primary cells: These cells are taken directly from a patient, cultured within a lab setting, and can accurately model unique genetic conditions and variability associated with neurodegenerative diseases. However, primary cells can be challenging to maintain and have limited reproducibility and growth.
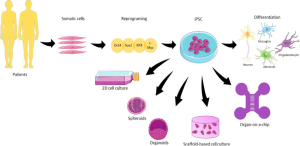
Figure 1. Graphical representation of iPSC use in neuronal cell culture from Pereira et al.
Computational Modelling
This is a unique form of disease modelling that, rather than relying directly on living systems, makes use of the abundance of data on neurodegenerative diseases to develop data-driven models that aim to predict and analyze disease progression. Computational disease modeling encompasses many approaches, each targeting different aspects of disease complexity. One example is multi‑modal modelling, which integrates physiological data across spatial and temporal scales. These studies often pair behavioural and cognitive assessments with brain imaging to build models that link symptoms to the disease’s physical progression. Their aim is to profile disease progression and improve diagnostics.
Jones et al. developed such a model, published in Nature Communications, in which they sought to create a model that maps the association between the dementia symptoms that are common in Alzheimer’s disease with physical degeneration in the brain. These models are providing a crucial link between physical and mental symptoms and are helping to better understand the variability of symptoms across these diseases.
Summary
With the growing prevalence of neurodegenerative disorders, there is an escalating need for disease models that are accurate, reproducible, and physiologically relevant. Continued refinement of in vivo, in vitro, and computational modelling platforms is providing researchers with critical insights into disease mechanisms and facilitating the rational design of novel therapeutics.
Related StressMarq products
StressMarq Biosciences supports leading neurodegeneration research with a broad range of high‑quality proteins for modelling Alzheimer’s, Parkinson’s, and related disorders. Our portfolio includes monomeric, oligomeric, and fibrillar forms of tau, alpha‑synuclein, and amyloid‑beta for disease modelling and preclinical drug discovery. Visit our product citations page to see how these tools are driving scientific progress.
References
- A computational model of neurodegeneration in Alzheimer’s disease. Jones et al., Nat Commun. 2022
- Advances in current in vitro models on neurodegenerative diseases. Pereira et al., Bioeng. Biotechnol. 2023
- Artificial miRNA mediated reduction of SNCA for the treatment of α-synucleinopathies. Elmer, B. et al., Mol Ther. 2025
- Astrocytic Sox9 overexpression in Alzheimer’s disease mouse models promotes Aβplaque phagocytosis and preserves cognitive function. Choi et al., Nat Neurosci. 2026
- Beyond the usual suspects: multi-factorial computational models in the search for neurodegenerative disease mechanisms. Khan & Iturria-Medina, Transl Psychiatry. 2024
- Bridging the gap: large animal models in neurodegenerative research. Eaton & Wishart, Mamm Genome. 2017
- Imaging plus X: multimodel models of neurodegenerative disease. Oxtoby & Alexander, Curr Opin Neurol. 2017
- In vitro Models of Neurodegenerative Diseases. Slanzi et al., Cell Dev. Biol. 2020

Leave a Reply