Investigating the Ectosomal Transport of Alpha Synuclein
The misfolding and intercellular transmission of alpha synuclein is central to the progression of Parkinson’s disease. Misfolded alpha synuclein acts as a seed, inducing the misfolding and aggregation of normal alpha synuclein in neighboring neurons. Mutations in the GBA1 gene, which encodes the enzyme β-glucocerebrosidase (GCase), are the most common genetic alterations linked to Parkinson’s disease (PD). GCase plays a crucial role in lipid metabolism by hydrolyzing glucosylceramides (GlcCer) into ceramide and glucose. GlcCer are sphingolipids found in the Golgi apparatus, plasma membrane, and lipid rafts.
Loss-of-function mutations in GBA1 reduce GCase activity, leading to the accumulation of GlcCer. This buildup contributes to the misfolding and aggregation of alpha synuclein, a hallmark of PD pathology. Notably, similar reductions in GCase activity and increases in GlcCer levels have also been observed in familial PD cases involving mutations in the LRRK2 gene, which encodes leucine-rich repeat kinase 2. Although the link between altered lipid metabolism and alpha synuclein transmission is not yet fully understood, extracellular vesicles have been proposed as a potential mechanism mediating this process.
Mechanisms of intercellular transport
Extracellular vesicles (EVs) are small, membrane-bound particles released by cells into the extracellular space. They include several subtypes that often overlap in size but differ in their functions and mechanisms of biogenesis. One such subtype, known as ectosomes, forms through outward budding from lipid rafts in the plasma membrane. These lipid rafts are rich in sphingolipids, which are found at elevated levels in Parkinson’s disease. Importantly, alpha synuclein has been observed to interact with these lipids, which in turn has been shown to promote its aggregation.
Researchers at the University of Alberta in Canada investigated whether ectosomes are the specific EV subtype responsible for the intercellular transmission of pathogenic alpha synuclein. As distinguishing EV subtypes after their release is technically challenging, Jacquemyn et al. utilized live-cell imaging to directly observe EV formation at the plasma membrane in real time, in a study published on bioRxiv.
Identifying vesicular species in vitro
In the later stages of Parkinson’s disease, cortical neurons in the cerebral cortex accumulate misfolded alpha synuclein aggregates. These inclusions, known as Lewy bodies (LBs), are commonly observed in PD pathology. To investigate how glucosylceramide influences ectosome shedding, researchers fluorescently labeled primary rat cortical neurons to visualize their plasma membranes. They engineered a construct in which a portion of CD58 was fused to the fluorescent tag mCherry (mCh). As CD58 binds glycosylphosphatidylinositol (GPI) on the plasma membrane, the resulting mCh-GPI construct anchored stably to the neuronal surface. Glucosylceramide itself was tagged with nitrobenzofurazan (NBD), enabling its visualization.
Live imaging of the labeled neurons treated with NBD-glucosylceramide revealed strong enrichment of the lipid at the plasma membrane, accompanied by an approximately 26-fold increase in vesicle budding compared with untreated controls. These vesicles were confirmed to be ectosomal in origin.
The team next examined whether inhibiting GCase activity would alter ectosome formation. Using the small-molecule inhibitor conduritol-β-epoxide (CBE), they observed by time-lapse imaging that GCase inhibition led to glucosylceramide accumulation and enhanced vesicle budding. Thus, reduced GCase activity, and the consequent rise in glucosylceramide levels, drove ectosome production in vitro.
In vivo live-cell imaging
To test whether the same phenomenon occurs in vivo, two-photon microscopy was used to image the cortices of live mice. Cortical neurons were labeled with the soluble fluorophore tdTomato, then injected with either NBD-glucosylceramide or a saline control. Consistent with the cell-culture findings, tdTomato-positive vesicles were seen budding from neurites in anesthetized mice. Super-resolution microscopy of fixed brain tissue further revealed fluorescent puncta, representing vesicles or protein aggregates, detached from labeled neurons.
The number of puncta increased markedly following NBD-glucosylceramide administration. Interestingly, certain puncta contained both NBD-glucosylceramide and tdTomato signal, while additional tdTomato-positive puncta were detected in surrounding neurons. Together, these results demonstrate that glucosylceramide promotes ectosome formation and that the released vesicles can be transferred to neighboring cells.
Implications of key mutations in Parkinson’s disease
A characteristic feature of Parkinson’s disease associated with GBA1 and LRRK2 mutations is reduced glucocerebrosidase activity, accompanied by elevated glucosylceramide levels. Since both factors promote ectosome shedding, the researchers designed an in vitro study to test whether PD neurons release more ectosomes.
Induced pluripotent stem cell (iPSC)-derived lines from PD patients carrying GBA1 mutations (N370S, L444P, or W378G) or LRRK2 mutations (G2019S or R1441H) were differentiated into dopaminergic neurons, a population known to degenerate early in PD. In comparison with established controls, these mutant neurons exhibited significantly reduced GCase activity and increased ectosome shedding, confirming that excessive vesicle release is a cellular feature of PD linked to impaired GCase function.
Importantly, restoring GCase activity in the mutant dopaminergic neurons reduced ectosome shedding to near-control levels, establishing a direct causal relationship between GCase activity and ectosome release.
Visualizing the ectosomal transport of pathogenic alpha synuclein with StressMarq’s neurodegenerative protein constructs
To examine whether the pathological spread of fibrils that induce misfolding of endogenous alpha synuclein is facilitated by ectosomal activity, rat cortical neurons were exposed to StressMarq’s commerically-available Alpha Synuclein Pre-formed Fibrils: ATTO 594 (catalog# SPR-322-A594) or untagged lab-produced alpha synuclein pre-formed fibrils and monomers. Both types of PFFs triggered time-dependent phosphorylation of alpha synuclein at Ser129, a post-translational modification closely associated with aggregation and disease pathology. By contrast, monomeric alpha synuclein had no such effect.
Live-cell super-resolution imaging revealed that labeled PFFs accumulated within ectosomes budding from the plasma membrane following NBD-glucosylceramide treatment. When neurons were treated with the GCase inhibitor, the fraction of vesicles containing labeled PFFs increased markedly from 30% to 60%. These findings suggest that glucosylceramide-induced ectosomes actively mediate the release of alpha synuclein from neurons.
Consistent with this, patient-derived dopaminergic GBA1-N370S neurons were also shown to secrete ectosomes containing alpha synuclein. Importantly, this alpha synuclein was phosphorylated, confirming its pathogenic state. Further experiments verified that the vesicles were indeed ectosomal in nature and, crucially, capable of transmitting toxicity to recipient neurons.
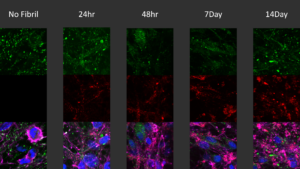
[Image from: StressMarq website.] Immunocytochemistry / Immunofluorescence analysis of human iPSC-derived neurons treated with StressMarq’s Alpha Synuclein Pre-formed Fibrils: ATTO 594 (2.5 µg, red) for up to 14 days. Cells seeded at 8k cells per well.
Summary
The experimental data presented in this study demonstrates that ectosomes are the extracellular vesicles responsible for the pathogenic spread of misfolded alpha synuclein, a central driver of neurodegeneration in Parkinson’s disease. Using live-cell imaging, Jacquemyn et al. identified ectosomes as carrier vehicles and demonstrated their formation to be tightly linked to dysregulated lipid metabolism. Specifically, inhibition of GCase was shown to elevate glucosylceramide levels and promote ectosome budding in both rodent and patient-derived models. These findings highlight lipid metabolism as a potential therapeutic target for limiting alpha synuclein propagation and its associated neurotoxicity, and suggest that ectosomal transport may be implicated across several neurodegenerative disease pathologies.
Related StressMarq products
StressMarq offers a wide range of neurodegenerative protein tools designed to advance disease modelling and preclinical drug discovery for the treatment of neurodegenerative diseases. To further support investigations into Parkinson’s disease mutations, StressMarq manufactures Alpha Synuclein E114C Mutant Pre-formed Fibrils: ATTO 488 (catalog# SPR-518-A488) and Alpha Synuclein A90C Mutant Monomers (catalog# SPR-478). Visit our website for detailed information and the latest scientific publications showcasing the use of our specialized alpha synuclein, tau, and amyloid beta pre-formed fibrils, oligomers, and monomers in cutting-edge research.
References
- Glucosylceramide induced ectosomes propagate pathogenic α-synuclein in Parkinson’s disease. Jacquemyn, J. et al., bioRxiv [Preprint]. 2025.

Leave a Reply