Assessing Membrane Permeability in Neurodegeneration
The plasma membrane is the outer boundary of the cell, controlling what enters and exits while maintaining cell homeostasis. It functions as a physical barrier to the extracellular environment, providing mechanical stability, and facilitating signal transduction and cell-to-cell communication. The loss of plasma membrane integrity is associated with neurodegenerative diseases such as Alzheimer’s (AD) and Parkinson’s disease (PD). Additionally, this loss has been implicated in the development of several types of cancer. However, the immune system also uses membrane disruption as a method to destroy invading pathogens.
Recently, this mechanism has been exploited to develop therapeutic compounds that can permeabilize the membranes of microbes that cause infectious disease. Therefore, the development of rapid and sensitive assays that measure membrane disruption is crucial for drug discovery and innovation.
Microscopy of synthetic lipid vesicles
In an ongoing collaboration between The University of Edinburgh and StressMarq Biosciences, Bąk et al. developed a highly sensitive, high-throughput single-molecule confocal assay to monitor Ca²⁺ transport in dye-loaded large unilamellar vesicles (LUVs). This serves as a readout of membrane permeabilization, and represents a significant improvement over prior methods. Previously, methods depended on spectrofluorometric detection of fluorescent dyes released from disrupted vesicles following treatment with a permeabilizing agent.
Such bulk measurement of vesicles lacks sensitivity and requires substantial quantities of active compound. Other microscopy-based methods also present limitations, as they genrally necessitate the use of especially large vesicles. In the case of total internal reflection fluorescence (TIRF) microscopy, vesicle immobilization is required to assess permeabilization at the single-molecule level.
Published in Angewandte Chemie International Edition, a prime chemistry journal of the German Chemical Society, the authors developed a unique approach combining single-molecule confocal microscopy with fast-flow microfluidics. This method utilizes easily produced synthetic lipid vesicles (LUVs), with fluorescence detected from within the vesicle itself. Upon binding to Ca2+, the encapsulated fluorescent dye (Fluo-8 or Cal-520) remains inside the LUV and is activated. When membrane disruption occurs, Ca2+ from the external environment enters the vesicle, triggering fluorescence. This process enables real-time monitoring of individual LUVs using fast-flow fluidics coupled with confocal microscopy. Furthermore, this method does not require vesicle immobilization, and detection may reach an impressive rate of 1,000 vesicles per minute.
Detection of ionophore and peptide activity
Ionophores play a crucial role in the transport of ions across biological membranes. To evaluate the assay’s performance, the researchers employed the Ca2+ ionophore ionomycin, a natural antibiotic that specifically transports calcium ions. The addition of CaCl2 to Fluo-8 dye‑loaded LUVs caused a small baseline change. This was likely from residual unencapsulated dye or leaky vesicles.
In contrast, adding ionomycin produced a dramatic, concentration-dependent increase in high‑intensity fluorescent bursts, reflecting Ca2+ transport into intact vesicles. The assay demonstrated considerable sensitivity, detecting ionomycin activity down to concentrations of 135 pM – corresponding to roughly eight ionomycin molecules per vesicle. With a sample consumption of only 100 μL per measurement, this method requires minimal material and is ideal for testing scarce or valuable compounds.
Next, Bąk et al. tested two antimicrobial peptides with distinct mechanisms of action and pore sizes. First, the researchers evaluated Alamethicin, which forms relatively small barrel-stave pores. At high alamethicin concentrations, the authors observed leakage of Fluo‑8 dye, prompting them to switch to Cal‑520 conjugated to 10 kDa dextran – a larger probe unable to pass through the small alamethicin pores. Using this dextran-conjugated dye, alamethicin displayed clear dose-dependent Ca2+ transport. These results demonstrated that the assay can effectively resolve small‑pore formation by selecting an appropriately sized reporter molecule to mitigate leakage.
In contrast, melittin forms wider, transient pores. As observed with alamethicin, melittin induced Fluo‑8 leakage at higher concentrations but facilitated measurable Ca2+ transport with the dextran‑tagged probe. In comparison with alamethicin, melittin displayed greater variance in activity. Melittin activity levels were consistent with previously reported variability in membrane assembly and pore-forming dynamics. These experiments illustrate how reporter selection (free dye vs dextran-conjugated dye) can differentiate between leakage and ion transport. In addition, reporter selection allows for mechanistic inferences regarding pore size and stability.
Exploring alpha synuclein aggregation & membrane disruption with StressMarq’s proteins for neurodegenerative disease research
The importance of this assay is also underscored by its application to alpha synuclein, whose aggregated species are implicated in Parkinson’s disease. Investigators incubated StressMarq’s Alpha Synuclein A90C Mutant Monomers (catalog# SPR-478) under aggregation‑favoring conditions and collected aliquots across a 72‑hour time course. When the authors added these protein samples to Fluo‑8 LUVs, early time points (0-4 hours) produced a decrease in event frequency, suggesting partial vesicle disruption or altered detection efficiency. Activity then increased between 8 and 24 hours, reaching a maximum at 24 hours before declining toward baseline at 48-72 hours.
To identify the membrane-permeabilizing species, the research team employed single‑aggregate visualization by enhancement (SAVE) using Thioflavin-T (ThT) and TIRF microscopy. Both aggregate counts and ThT fluorescence intensity increased substantially after 24 hours, indicating the presence of oligomeric species coinciding with peak permeabilization activity. These findings support a model in which early oligomeric alpha synuclein species are the most membrane‑active, while the subsequent formation of large aggregates and fibrils reduces the pool of permeabilizing oligomers and lowers measurable activity. This time‑resolved, single‑vesicle assay thus provides a sensitive and simplified method of linking aggregate population dynamics to functional membrane disruption.
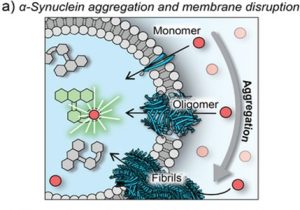
Figure 1. Schematic representation of membrane permeabilization with monomeric and aggregated α‐synuclein. (Figure taken from Bąk. K. et al, 2025 used under license CC BY 4.0).
Summary
The findings reported in this study introduce an ultrasensitive platform that bridges single‑molecule sensitivity with high throughput to quantify membrane permeabilization in freely flowing LUVs. By detecting individual vesicle transit events at rates approaching 1,000 vesicles per minute and achieving a detection limit as low as 135 pM, Bąk. K. et al. present a novel method that overcomes the key limitations of traditional bulk fluorimetry and TIRF-based single‑vesicle assays. Moreover, it minimizes material requirements while resolving activity down to just a few molecules per vesicle. These capabilities have clear relevance for antimicrobial drug discovery and for investigating aggregate-mediated membrane damage in neurodegenerative diseases.
Related StressMarq products
Trusted by scientists worldwide for exceptional quality and consistency, StressMarq offers a comprehensive range of products for neurodegenerative research. Alongside a diverse portfolio of alpha synuclein protein constructs, StressMarq offers a broad selection of antibodies, including the recently launched Alpha Synuclein N-Terminal Antibody (catalog# SMC-621). For real-time fluorescence monitoring, Alpha Synuclein Pre-formed Fibrils: ATTO 594 (catalog# SPR-322-A594) are also available. Visit our website to learn more, and to explore the latest scientific publications using our specialized alpha synuclein, tau, and amyloid beta proteins.
References
1. A single-molecule liposome assay for membrane permeabilization. Bąk, K.M. et al., Angew Chem Int Ed Engl. 2025.


Leave a Reply