Conformational Nanobodies Targeting Tau Aggregates
Neurodegenerative diseases represent a growing global health burden, with their prevalence steadily increasing alongside the aging population. Alzheimer’s disease (AD) is one of the most common instances, and is characterized by the abnormal accumulation of misfolded tau and amyloid beta protein plaques, which trigger neuronal inflammation, dysfunction, and ultimately cell death.
To date, the FDA has approved three monoclonal antibody therapies targeting amyloid beta plaques. However, effectively targeting tau fibrillar aggregates remains a critical goal, as these aggregates are believed to facilitate the spread of disease-modifying tau between distinct brain regions.Within this context, tau conformational nanobodies have emerged as a promising therapeutic strategy for Alzheimer’s disease and other tauopathies.
Nanobodies, which are composed solely of the variable domains of heavy-chain antibodies and lack light chains, offer several advantages over traditional monoclonal antibodies. Their small size, high stability, and strong antigen-binding affinity make them particularly well-suited for targeting pathological proteins in neurodegenerative diseases. Despite these advantages, the development of tau-specific nanobodies that selectively bind pathological fibrillar aggregates while sparing physiologically important monomeric tau has been limited by the lack of effective selection and sorting methods. This is especially true given the large and complex conformation of the tau protein.
In a significant advancement, Zupancic et al. have streamlined the nanobody discovery process by employing quantitative fluorescence-activated cell sorting (FACS) of yeast-displayed libraries against tau fibrillar aggregates. This innovative approach enabled the identification of nanobodies with high specificity for pathological tau. Moreover, the method allows for the generation of conformational nanobodies in various formats, including bispecific and multivalent constructs, expanding their potential for both diagnostic and therapeutic applications in neurodegenerative diseases.
Generating high-specificity tau nanobodies
Overcoming the longstanding challenges of generating conformational nanobodies against large proteins such as tau, which has 441 amino acids in its longest isoform, requires a novel and strategic approach. Researchers at the University of Michigan addressed this challenge by employing quantitative flow cytometric sorting of yeast-displayed nanobody libraries, enabling the direct selection of nanobodies that bind specifically to tau fibrils.
To initiate nanobody production, llamas were immunized with StressMarq’s Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (catalog# SPR-329). This tau isoform is the longest and includes the P301S mutation, which is associated with enhanced aggregation and behavioral abnormalities resembling dementia symptoms. The immunization successfully elicited increased serum antibody levels that bound to immobilized tau fibrils, as confirmed by flow cytometry.
Further optimization was achieved by immunizing llamas with StressMarq’s Truncated Tau Fragment (AA297–391) (dGAE) Pre-formed Fibrils (catalog# SPR-461). This truncated fragment contains the minimal structural elements required for tau aggregation into neurofibrillary tangles, a hallmark of AD. Immunization with the truncated tau fragment enhanced antibody production and improved serum antibody binding to both full-length and truncated tau fibrils, further validating the approach.
Improving selection and sorting
To initiate the sorting of nanobodies, researchers employed StressMarq’s Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (catalog# SPR-329) in combination with magnet-activated cell sorting (MACS). For the enrichment step, functionalized magnetic beads conjugated with these full-length tau PFFs were used to selectively isolate nanobodies that specifically recognized tau fibrils.
To further enhance enrichment, the team incorporated fluorescence-activated cell sorting (FACS) using quantum dots. These nanocrystals emit strong, stable fluorescence, allowing for highly sensitive detection. By immobilizing tau fibrils onto quantum dots, researchers could precisely sort nanobodies based on their binding affinity using FACS.
The nanobodies selected through this process demonstrated conformational specificity for tau fibrillar aggregates, exhibiting minimal binding to monomeric tau. This specificity is critical for distinguishing pathological tau species from their physiological counterparts, reinforcing the potential of these nanobodies for diagnostic and therapeutic applications.
Verification of nanobody specificity
The next phase of the study focused on verifying whether the selected nanobodies could selectively recognize and bind pathological tau aggregates in both mouse and human samples. To facilitate detection, the nanobodies were expressed as Fc-fusion proteins, incorporating the Fc region of human IgG1.
Among the candidates, one nanobody—WA2.22—was successfully identified for its ability to recognize tau aggregates in brain tissue from the widely studied P301S transgenic mouse model, which overexpresses the human tau P301S mutation associated with tauopathies.
Importantly, WA2.22 also demonstrated strong reactivity in human brain tissue samples from patients with tauopathies. The staining pattern observed with WA2.22 was comparable to that of Zagotenemab, a clinical-stage tau conformational antibody. Both WA2.22 and Zagotenemab produced similar aggregate staining patterns across multiple regions of the analyzed brain sections, confirming the nanobody’s conformational specificity and diagnostic potential.
To conclude the study, it was essential to demonstrate that the selected tau fibril nanobodies exhibited low non-specific binding and high specificity to their intended targets—key requirements for future diagnostic or therapeutic applications. The nanobodies generated through the improved sorting and selection methods met these criteria, showing minimal off-target interactions. Their performance was comparable to that of highly specific clinical-stage antibodies, reinforcing their potential for further development in the detection and treatment of tau-related neurodegenerative diseases.
StressMarq reagent toolbox of neurodegenerative constructs improves conformational nanobodies generation
Conformational nanobodies are critical for distinguishing between physiological monomeric tau and pathological fibrillar tau aggregates found in Alzheimer’s and Parkinson’s disease. The scarcity of such nanobodies has largely stemmed from limitations in traditional nanobody generation and selection methods.
In a breakthrough study, Zupancic et al. developed a novel approach to directly isolate nanobodies from immune libraries using quantitative fluorescence-activated cell sorting (FACS) of yeast-displayed libraries against tau aggregates conjugated to quantum dots. This method eliminated the need for secondary screening, significantly streamlining the discovery process.
Key to this success were StressMarq’s Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (catalog# SPR-329) and Truncated Tau Fragment (AA297–391) (dGAE) Pre-formed Fibrils (catalog# SPR-461), which were instrumental in both the initial immunization and the subsequent sorting and selection steps.
This improved protocol for generating tau conformational nanobodies that specifically target aggregated fibrillar tau holds significant promise for advancing therapeutic and diagnostic development in neurodegenerative disease research.
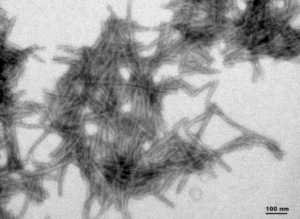
[Image from: StressMarq website] TEM imaging of StressMarq’s Truncated Tau Fragment (AA297–391) (dGAE) PFFs (catalog# SPR-461).
Summary
Developing conformational nanobodies against large, complex proteins implicated in neurodegenerative diseases is essential for advancing both diagnostic and therapeutic strategies. This study represents a major step forward in this effort. By enhancing the nanobody sorting and selection process through quantitative fluorescence-activated cell sorting (FACS) of yeast-displayed libraries against tau aggregates conjugated to quantum dots, the researchers introduced a more efficient and targeted discovery pipeline.
These improved protocols have the potential to revolutionize the diagnosis and treatment of neurodegenerative diseases by enabling the generation of nanobodies with high specificity for pathological protein conformations—paving the way for more precise and effective interventions.
Related StressMarq products
StressMarq’s portfolio of products offers a powerful and versatile toolbox for advancing neurodegenerative disease research, particularly in the development of conformational nanobodies targeting complex disease-related proteins. With a comprehensive range of oligomeric, monomeric, and fibrillar forms of tau, amyloid beta, and alpha synuclein, StressMarq is dedicated to supporting innovation in the field.
Explore our website to discover the latest scientific publications and breakthroughs made possible using our products.
References
- Quantitative flow cytometric selection of tau conformational nanobodies specific for pathological aggregates. Zupancic, J. et al. Front Immunol. 2023.

Leave a Reply