Alpha Synuclein Fibrillar Constructs
This is an updated version of a previously published article.
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the misfolding and accumulation of alpha synuclein. Aggregated alpha synuclein is a key protein in PD pathogenesis and different forms of the protein can be used to develop disease models that reproduce the pathological features of this disorder. Alpha synuclein can aggregate generating soluble oligomers and insoluble fibrils. Misfolded alpha synuclein aggregates are toxic and lead to neurodegeneration.
StressMarq Biosciences offers a wide variety of monomeric, oligomeric and fibrillar alpha synuclein constructs for PD research. These preparations have different characteristics and properties.
Alpha Synuclein Monomers
In chemistry, a monomer refers to a single molecule that can combine to form polymers. In the context of alpha synuclein, a monomer refers to a single alpha synuclein protein (~14 kDa). Studies have shown that alpha synuclein exists predominantly as a disordered monomer in the cytosol. Non-toxic alpha synuclein monomers rapidly aggregate into toxic oligomers, protofibrils, and ultimately large fibrils that can seed further aggregation. During this process, alpha synuclein adopts multiple conformations and there is a dynamic equilibrium between monomeric, oligomeric and fibrillar forms.
Type 1 monomers aggregate to form Type 1 pre-formed fibrils.
Type 2 monomers aggregate to form Type 2 pre-formed fibrils.
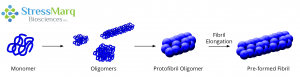
Monomers aggregate to form oligomers, protofibril oligomers, and ultimately lengthen into fibrils.
Alpha Synuclein Oligomers

An oligomer is composed of repeating monomer units and has quaternary structure. When monomers aggregate, they can form a variety of different sizes and types of oligomers. Oligomeric species usually have a roughly spherical structure (~25nm diameter). In Parkinson’s disease alpha synuclein oligomers seem to induce significantly higher neuronal toxicity compared to fibrils
Kinetically Stable Alpha Synuclein Oligomers: These oligomers are generated from Type 2 monomers without the addition of any inducers or inhibitors and remain stable after a freeze-thaw cycle and when incubated at 37°C for 14 days. They have been shown to be toxic to dopaminergic neurons and induce pS129 pathology.
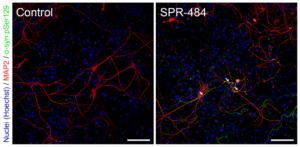
Representative immunohistochemistry images of Parkinson’s-associated pSer129 pathology induced in rat primary dopaminergic cells by Human Alpha Synuclein Oligomers (Kinetically-Stable) (catalog# SPR-484).
Dopamine-stabilized Alpha Synuclein Oligomers: The neurotransmitter dopamine can inhibit the aggregation of alpha synuclein into fibrils and stabilize oligomers. Dopamine-stabilized oligomers show toxicity in primary mouse neurons. They may also inhibit monomer fibrillization.
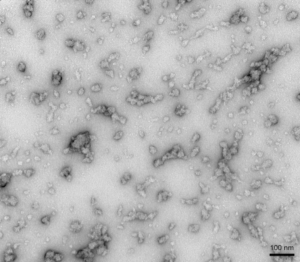
TEM of Human Alpha Synuclein Oligomers (Dopamine HCL Stabilized) (catalog# SPR-466).
EGCG-stabilized Alpha Synuclein Oligomers: The flavonoid epigallocatechin gallate (EGCG) has been shown to stabilize alpha synuclein in its oligomeric form. These oligomers are thought to be non-toxic and do not seed monomer aggregation in Thioflavin T assays.
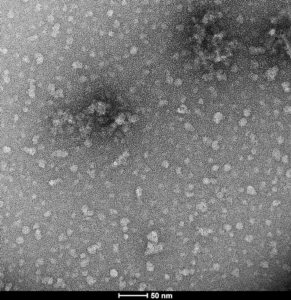
TEM of Human Alpha Synuclein Oligomers (Epigallocatechin gallate [EGCG] Stabilized) (catalog# SPR-469).
Alpha Synuclein Filaments
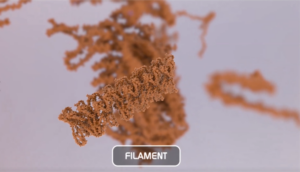
Oligomers further aggregate into soluble protofibrils or filaments. Filaments can undergo a structural change from an alpha-helix to a beta-sheet and form insoluble fibrils. Under the electron microscope (EM), filaments look similar to mature fibrils but they have different properties.
Alpha Synuclein Filaments do not generate pSer129 pathology or toxicity and do not show seeding capability in SH-SY5Y cells.
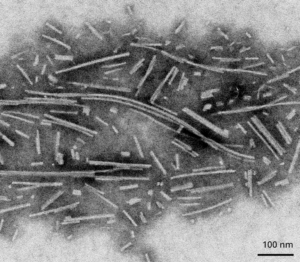
TEM of Active Human Alpha Synuclein Protein Filaments (catalog# SPR-450).
Alpha Synuclein Pre-formed Fibrils (PFFs)

Alpha synuclein fibrils are formed through the polymerization of monomeric peptides into long fibers. These fibrils are insoluble aggregates characterized by an extended beta-sheet secondary structure.
Alpha synuclein fibrils can be isolated from PD patient brains and used to seed Lewy body pathology in models. These fibrils can also be manufactured recombinantly.
Fibrils can be injected into cell cultures or directly into rodents and are transported within the cells. Active alpha synuclein fibrils are able to seed their monomeric alpha synuclein, which aggregates into more complex structures. Pathology develops much faster than in transgenic models. Certain mutants such as A53T or S87N promote aggressive aggregation.
StressMarq offers different types of fibrils that look similar under the electron microscope (EM), but they are different in terms of seeding and toxicity.
Human Alpha Synuclein PFFs (Type 1): The human PFFs have been shown to induce pSer129 pathology in iPSC-derived neurons within 7 days and in primary rat neurons in 14 days. Experiments have also shown that the human PFFs are taken up by SH-SY5Y cells and transmitted to neuronal iPSCs within 14 days. In vivo, these fibrils have been validated in Sprague-Dawley Rat brain and induce alpha synuclein pathology 30 days post-injection.
StressMarq also offers fluorescent labelled Human Alpha Synuclein PFFs which allow for tracking fibril activity in vitro. The conjugation does not impact seeding capability of the fibrils.
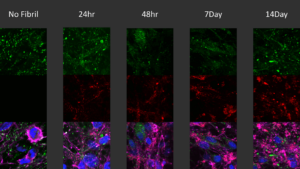
Immunocytochemistry / Immunofluorescence analysis of human iPSC-derived neurons (Stem Cell Catalog Code ASE-9321KF) treated with 2.5µg Human Alpha Synuclein Pre-formed Fibrils: ATTO 594 (Type 1) (catalog# SPR-322-A594) for up to 14 days.
Human Alpha Synuclein PFFs (Type 2) : Type 2 PFFs have been seen to induce toxicity in vitro in primary rat cortical neurons and alpha synuclein pathology much more slowly than Type 1 PFFs.
Human Alpha Synuclein PFFs: Biotinylated (C-Terminus): Biotinylation of purified alpha synuclein allows for many biological applications, such as monitoring and detection using streptavidin-based conjugates. A 15 amino acid tag on the C-terminal tail of the alpha synuclein protein facilitates site-specific covalent biotinylation. Biotinylated alpha synuclein fibrils seed fibril formation of biotinylated monomers over 72 hours.
Mouse Alpha Synuclein PFFs (Type 1) : The mouse PFFs induce pathology and toxicity in primary rat and mouse hippocampal neurons. In vivo, they have been shown to induce pathology after injected in Sprague-Dawley Rats and C57/BL6 mice.
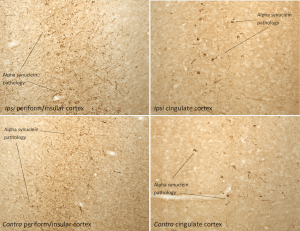
Immunohistochemistry analysis of rat brain injected with Mouse Alpha Synuclein Pre-formed Fibrils (Type 1) (catalog# SPR-324). Species: Female Sprague-Dawley Rat. Alpha synuclein pathology is seen in the periform/insular cortex and the cingulate cortex on both the same (ipsi) and opposite (contra) sides as the injection sites.
Rat Alpha Synuclein PFFs: The Rat Alpha Synuclein monomer is very active and able to form more beta-sheet structure alone than with the combination of monomer and fibril. In combination, the fibril is the majority of the seed.
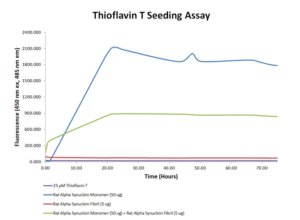
Thioflavin T seeding assay using Rat Alpha Synuclein Pre-formed Fibrils (catalog# SPR-482).
Alpha Synuclein Type 1 and Type 2 – What’s the Difference?
Although Human Type 1 (catalog# SPR-321) and Type 2 (catalog# SPR-316) monomers are identical with respect to sequence, they have different expression conditions. Type 1 is naturally low endotoxin (<5 EU/mL) while Type 2 undergoes endotoxin removal to ensure that it is <5 EU/mL, prior to fibrilization. As a result, their beta-sheet content differs leading to distinct properties in seeding activity and pSer129 pathology post-fibrillization.
Specifically, Type 1 fibrils (catalog# SPR-322) have been shown to produce a more robust Thioflavin T response, with a much higher signal in seeding assays. On the other hand, Type 2 fibrils (catalog# SPR-317) have a weaker Thioflavin T signal and some secondary structure differences.
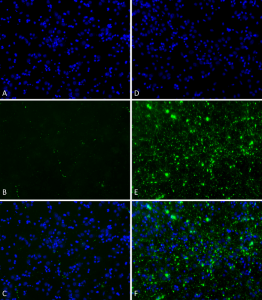
Primary rat hippocampal neurons show Lewy body inclusion formation when treated with Human Alpha Synuclein Pre-formed Fibrils (Type 1) (catalog# SPR-322) at 4 µg/ml (D-F), but not when treated with Human Alpha Synuclein Pre-formed Fibrils (Type 2) (catalog# SPR-317) at 4 µg/ml (A-C).
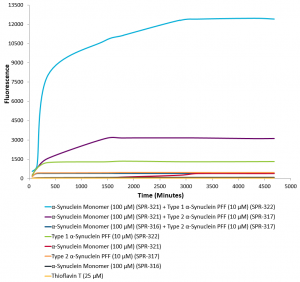
Thioflavin T Aggregation Assay. Type 1 Human Alpha Synuclein PFFs (catalog# SPR-322) show greater seeding capabilities than Type 2 Human Alpha Synuclein PFFs (catalog# SPR-317).
Alpha Synuclein Mutant PFFs
A53T mutant: The A53T mutation is a missense point mutation where alanine is replaced by threonine at the 53rd amino acid. This mutation has been linked to early-onset Parkinson’s Disease and increased rates of alpha synuclein fibrillization. StressMarq’s A53T mutant PFFs generate rapid aggregation and pSer129 pathology.
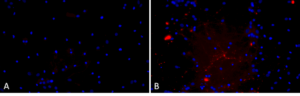
Primary rat hippocampal neurons show Lewy body inclusion formation when treated with Human Alpha Synuclein A53T Pre-formed Fibrils (catalog# SPR-326) (B) but not when treated with a media control (A).
S87N Mutant: Human alpha synuclein S87N mutant (HuS87N) has Ser87 mutated to the equivalent mouse residue Asn87, effectively making it a human-mouse chimeric protein. According to literature, S87N substitution in human α-syn substantially accelerates fibrilization rates in vitro. Also, Chimeric HuS87N fibrils show enhanced induction of α-syn pathology greater than both Human WT and Mouse WT fibrils in mice neuron cultures. Therefore, Human S87N is a good construct for inducing robust endogenous α-syn seeding and pathology in wild-type mice/cultures.
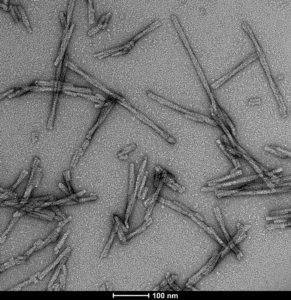
TEM of Human Alpha Synuclein S87N fibrils (catalog# SPR-500). Negative stain transmission electron microscopy images acquired at 80 Kv on carbon coated 400 mesh copper grids using phosphotungstic acid and uranyl acetate stain. Scale bar = 100 nm.
TNG (A53T, S87N, N103G) Mutant: TNG mutant (HuTNG) is a triple mutant containing Ala53 mutated to the equivalent mouse residue Thr53, Ser87 mutated to the equivalent mouse residue Asn87, and Asn103 mutated to the equivalent mouse residue Gly103, effectively making it a human-mouse chimeric protein. Chimeric HuTNG fibrils show enhanced induction of α-syn pathology greater than both Human WT and Mouse WT fibrils after single unilateral injection into the dorsal striatum in mice. Therefore, HuTNG is a good construct for inducing robust endogenous α-syn seeding and pathology in wild-type mice.
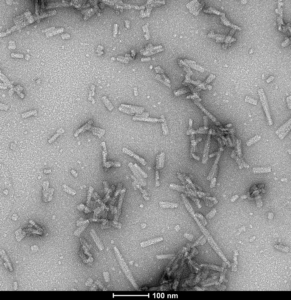
TEM of human alpha synuclein TNG (A53T, S87N, N103G) fibrils (catalog# SPR-504). Negative stain transmission electron microscopy images acquired at 80 Kv on carbon coated 400 mesh copper grids using phosphotungstic acid and uranyl acetate stain. Scale bar = 100 nm.
S129A Mutant: Elevated levels of phosphoserine 129 (pS129) on alpha synuclein has long been considered a hallmark of Parkinson’s disease and other synucleinopathies. Alpha synuclein S129A mutant monomers and fibrils cannot be phosphorylated at position 129, and therefore can be utilized to study phospho-S129-independent biology and pathology. Further, this material can be used to confirm induction of endogenous pS129 pathology in disease models.
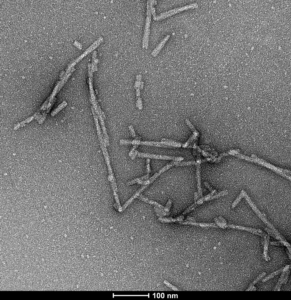
TEM of human alpha synuclein S129A fibrils (catalog# SPR-506). Negative stain transmission electron microscopy images acquired at 80 Kv on carbon coated 400 mesh copper grids using phosphotungstic acid and uranyl acetate stain. Scale bar = 100 nm.
E114C Mutant Conjugated to ATTO-488: These fibrils are formed from 10% fluorescently tagged E114C mutants & 90% wild-type monomers. The E114C mutation facilitates a single site-specific conjugation with Atto-488 maleimide that avoids any hindrance upon fibrilization or cell entry that may be conferred by non-specific lysine targeting conjugations. Atto-488 maleimide dye is a useful tool for identifying cell entry, as the addition of Trypan Blue to cultures prior to imaging will quench fluorescence of extracellular Atto-488 conjugated alpha synuclein. The E114C-ATTO 488 Pre-Formed fibrils are an excellent tool for studying cell entry and localization, with demonstrated entry into neurons post-Trypan Blue quenching.
N-Terminal Acetylated: Alpha synuclein purified from both normal and pathological brain tissue is N-acetylated and this post-translational modification affects alpha synuclein stability and toxicity.
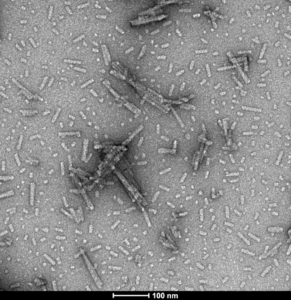
TEM of Human N-Terminal Acetylated Alpha Synuclein Pre-formed Fibrils (catalog# SPR-332).
Alpha Synuclein and Tau Co-Polymer Fibrils (Mixed Fibrils/PFFs)
StressMarq’s co-polymer fibrils are developed by co-incubating monomers together to form fibrils that contain both Tau and Alpha Synuclein proteins within a single fibril. Recombinant tau and alpha synuclein co-polymer fibrils have demonstrated a more widespread transmission of induced pathology in a rodent model of tauopathies compared to pure tau or alpha synuclein fibrils alone. These co-polymer fibrils have also shown enhanced alpha synuclein aggregation in vitro, and more severe alpha synuclein pathology and Parkinson’s disease-like symptoms in mice.
Human Tau-352 (fetal 0N3R) and Human Alpha Synuclein Co-Polymer Fibrils: Tau 0N3R, the shortest isoform of tau, is expressed in the fetal brain during neurogenesis and has been shown to be more prone to form oligomers in vitro. Tau-352 (fetal 0N3R) and Alpha Synuclein Co-polymer Fibrils seed fibril formation of both alpha synuclein monomers and of a mixture of alpha synuclein and fetal tau-352 monomers over 72 hours.
Human Tau-441 (2N4R) and Human Alpha Synuclein Co-Polymer Fibrils: The tau 2N4R isoform is expressed in the adult brain but is absent from the fetal brain. Tau-441 (2N4R) and Alpha Synuclein Co-polymer Fibrils seed fibril formation of both alpha synuclein monomers and of a mixture of alpha synuclein and tau-441 (2N4R) monomers over 72 hours.
Rigorous Quality Control
StressMarq’s quality control testing for neuroproteins includes:
- Sedimentation assays to ensure that most of the monomer was converted to fibril
- EM/AFM imaging to verify fibril formation
- Thioflavin T assay to assess seeding capability of the fibrils
- SDS-PAGE to ensure protein purity
- Sterility check
- Endotoxin testing
Product Citations
Many of StressMarq’s monomeric, fibrillar and oligomeric alpha synuclein preparations have been cited in research publications. Certain products have also been validated in both in vitro and in vivo studies by various StressMarq collaborators.


Leave a Reply