| Product Name | Niclosamide |
| Description |
Autophagy inducer |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 50-65-7 |
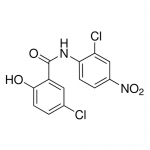
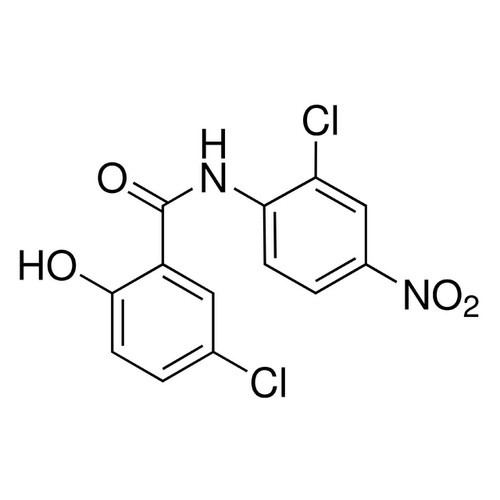
| Molecular Formula | C13H8Cl2N2O4 |
| Molecular Weight | 327.12 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inducer |
| Solubility | Soluble to 25 mM in ethanol and to 10 mM in DMSO |
| Source | Synthetic |
| Appearance | Pale yellow solid |
| SMILES | C2=C(C(NC1=C(C=C([N+](=O)[O-])C=C1)Cl)=O)C(=CC=C2Cl)O |
| InChI | InChI=1S/C13H8Cl2N2O4/c14-7-1-4-12(18)9(5-7)13(19)16-11-3-2-8(17(20)21)6-10(11)15/h1-6,18H,(H,16,19) |
| InChIKey | RJMUSRYZPJIFPJ-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Not WHMIS controlled. Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. |
| Cite This Product | Niclosamide (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-406) |
Biological Description
| Alternative Names | 5-Chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide |
| Research Areas | Autophagy, Cancer |
| PubChem ID | 4477 |
| Scientific Background | Niclosamide is an anthelmintic drug that has gained attention for its ability to modulate multiple signaling pathways, including Wnt/β-catenin, mTOR, and NF-κB. In neuroscience, niclosamide is investigated for its neuroprotective and anti-inflammatory properties. It has shown efficacy in reducing neuroinflammation and oxidative stress in models of neurodegenerative diseases. By targeting key regulatory pathways, niclosamide offers insights into the molecular mechanisms of neuronal survival and immune modulation. Its repurposing potential makes it a promising candidate for therapeutic development in neurodegeneration. |
| References | 1. Pan J.X., et al. (2012) Chin J Cancer. 31(4): 178-84. |

Reviews
There are no reviews yet.