| Product Name | Taxol |
| Description |
Microtubule stabilizer |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 33069-62-4 |
| Molecular Formula | C47H51NO14 |
| Molecular Weight | 853.9 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inducer |
| Solubility | Soluble in DMSO, ethanol or acetonitrile |
| Source | Synthetic |
| Appearance | White solid |
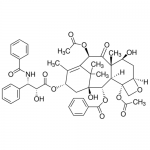
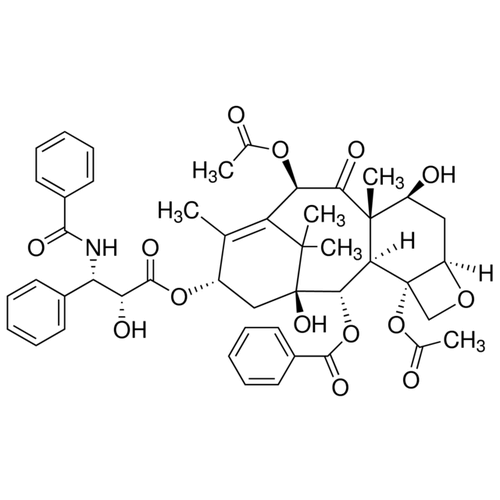
| SMILES | CC1=C2[C@@]([C@]([C@H]([C@@H]3[C@]4([C@H](OC4)C[C@@H]([C@]3(C(=O)[C@@H]2OC(=O)C)C)O)OC(=O)C)OC(=O)C5=CC=CC=C5)(C[C@@H]1OC(=O)[C@H](O)[C@@H](NC(=O)C6=CC=CC=C6)C7=CC=CC=C7)O)(C)C |
| InChI | InChI=1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20- |
| InChIKey | RCINICONZNJXQF-MZXODVADSA-N |
| Safety Phrases |
Classification: Harmful. May be harmful if inhaled, swallowed or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection Risk Phrases: R62- Possible risk of impaired fertility R68- Possible risk of irreversible effects Hazard Phrases: H318-H361f-H341-H332-H312-H302-H335-H315-H334-H317 Precautionary Phrases: P280-P305+P351+P338-P260-P261-P342+P311 |
| Cite This Product | Taxol (StressMarq Biosciences, Canada, Cat # SIH-239) |
Biological Description
| Alternative Names | (2α,5β,7β,10β,13α)-4,10-Diacetoxy-13-{[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate, Paclitaxel |
| Research Areas | Apoptosis, Cancer |
| PubChem ID | 36314 |
| Scientific Background | Paclitaxel, commonly known as Taxol, is a mitotic inhibitor that promotes and stabilizes tubulin polymerization, leading to cell cycle arrest. In neuroscience, paclitaxel has been explored for its ability to modulate microtubule dynamics, which are critical for axonal transport and neuronal structure. Its neurotoxic effects have also made it a model compound for studying chemotherapy-induced peripheral neuropathy. Additionally, paclitaxel induces apoptosis through caspase-10 activation, providing insights into programmed cell death mechanisms relevant to neurodegenerative disease models. |
| References |
1. Wani M., Taylor H., Wall M., Coggon P., and McPhail A. (1971) J Am Chem Soc. 93(9): 2325-2327. 2. Park S.J., et al. (2004) J Biol Chem. 279(49): 51057-51067. |

StressMarq Biosciences :
Based on validation through cited publications.