| Product Name | Tafamidis |
| Description |
Transthyretin Dissociation Inhibitor |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 594839-88-0 |
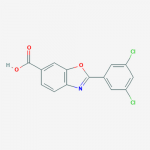
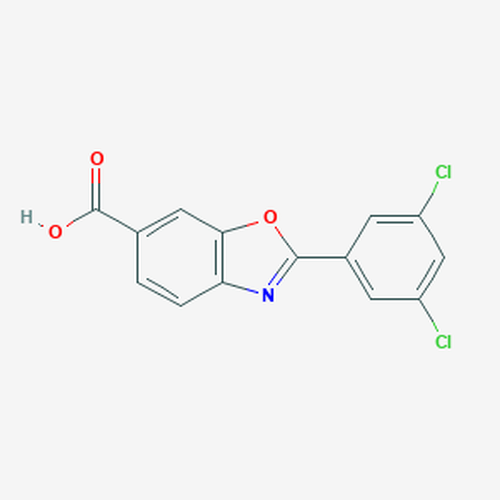
| Molecular Formula | C14H7Cl2NO3 |
| Molecular Weight | 308.1 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (25 mg/ml, warm) |
| Source | Synthetic |
| Appearance | Off-white powder |
| SMILES | C1=CC2=C(C=C1C(=O)O)OC(=N2)C3=CC(=CC(=C3)Cl)Cl |
| InChI | InChI=1S/C14H7Cl2NO3/c15-9-3-8(4-10(16)6-9)13-17-11-2-1-7(14(18)19)5-12(11)20-13/h1-6H,(H,18,19) |
| InChIKey | TXEIIPDJKFWEEC-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Danger- Irritant and Health Hazards GHS Hazard Statements: H315: Causes skin irritation H319: Causes serious eye irritation H335: May cause respiratory irritation H360: May damage fertility or the unborn child Precautionary Statements: P201, P202, P261, P264, P271, P280, P281, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 |
| Cite This Product | Tafamidis (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-584) |
Biological Description
| Alternative Names | 2-(3,5-Dichlorophenyl)-1,3-Benzoxazole-6-Carboxylic Acid, FX-1006, 2-(3,5-Dichlorophenyl)-6-benzoxazole carboxylic acid |
| Research Areas | Alzheimer's Disease, Axon Markers, Cell Markers, Cell Signaling, Cytoskeleton, Microtubules, MT Associated Proteins, Neurodegeneration, Neuron Markers, Neuroscience, Tangles & Tau |
| PubChem ID | 11001318 |
| Scientific Background |
Tafamidis is a pharmacological chaperone that stabilizes the tetrameric form of transthyretin (TTR), preventing its dissociation into monomers that can misfold and aggregate. While its primary clinical application is in delaying peripheral nerve damage in familial amyloid polyneuropathy (FAP), Tafamidis is increasingly recognized for its relevance in systemic amyloidosis research. Although not widely studied in neurodegenerative disease models, its mechanism—preventing amyloid fibril formation—parallels pathological processes in Alzheimer’s and other protein misfolding disorders. Tafamidis serves as a model compound for exploring amyloid stabilization strategies and may inform future therapeutic approaches targeting neurodegenerative amyloidoses. |
| References | 1. Said G, Grippon S, Kirkpatrick P. (2012). Nature Reviews. Drug Discovery. 11(3): 185–186. |

Reviews
There are no reviews yet.