Detecting Amyloid Beta: An Antibody Resource
As the first disease-modifying therapeutics for Alzheimer’s disease (AD) begin to receive approval for use in humans by pharmaceutical regulatory bodies, researchers are aiming to build on the success of Aducanumab, Lecanemab, and Donanemab by developing more therapeutic antibodies targeting amyloid beta (Aβ). Accumulation of amyloid beta plaques in the brain is a key indicator of Alzheimer’s disease, an incurable neurodegenerative disorder and the most common cause of dementia globally.
In AD pathology, the progressive accumulation of insoluble and toxic amyloid beta plaques alongside tau neurofibrillary tangles (NFTs) results in cell death and loss of normal brain function. As neuronal networks are progressively disrupted, symptoms may range from memory loss, deterioration of language and problem-solving skills and mood swings, to sleep disturbances, hallucinations, restlessness, and changes in appetite and weight.
Epitope mapping
Scientists from the Rudolf Virchow Center for Integrative and Translational Bioimaging at Julius-Maximilians-Universität (JMU) in Würzburg, Germany have published new research that discusses the exact binding epitopes of 20 antibodies directed against amyloid beta. This panel of antibodies includes research antibodies and biosimilars to Aducanumab, Lecanemab, and Donanemab. Extensive epitope mapping of these antibodies was conducted, incorporating factors such as truncations, familial Alzheimer’s disease mutations, and post-translational modifications (PTMs). Their reactivity with AD-associated structural variants, including oligomeric and fibrillar amyloid beta, was also assessed, with the goal of understanding how epitope alterations affect antibody interactions. The final antibody screening data may serve as a valuable resource for advancing investigations into the mechanisms underlying amyloid beta-antibody interactions.
Screening antibody candidates
Amyloid precursor protein (APP), a conserved transmembrane protein, is sequentially cleaved under physiological conditions by beta- and gamma-secretase, generating Aβ peptides. However, the conserved soluble peptides found in human cerebrospinal fluid (Aβ 1-37, Aβ 1-38, Aβ 1-39, Aβ 1-40 and Aβ 1-42) differ from those isolated from amyloid plaques. Amyloid plaques have been shown to contain several amyloid beta peptide variants that contain post-translational modifications and mutations, and can vary in length due to truncations or elongations. Many PTMs are located in the flexible N-terminal region around residues 1-16. It is thought that these variations may contribute to the pathophysiological aggregation of amyloid beta and plaque formation.
To begin the study, a panel of 20 antibodies central to AD research from a mixture of commercial and academic sources was used to carry out a pre-screen on a 60-peptide library derived from APP. This library of unmodified peptides – 15 amino acids long and overlapping by 14 amino acids – was immobilized on cellulose discs and probed by the 20-antibody panel in a microarray binding assay. The initial pre-screen provided a good starting point for further microarray analysis and other complementary assays.
Antibody selection
Subsequently, synthetically modified peptide libraries with post-translational modifications, mutations and truncations were employed to identify antibodies specific for several Aβ variants. These included N-terminal region-specific antibodies, antibodies detecting truncated peptide sequences and multiple post-translational modifications including phosphorylated serine residues and pyroglutamate (pE). Loss or gain of antibody binding due to mutations was also observed. In particular, the H6R mutation associated with familial AD was shown to abolish binding of Aducanumab, which has novel implications for the strategic treatment of AD patients.
Utilizing StressMarq’s Amyloid Beta 1-42 Constructs in the Assessment of Antibody Variant Binding
Next, the biosimilars were further characterized by their specificity for the most prevalent structural forms of Aβ 1-42. StressMarq’s Amyloid Beta 1-42 Oligomers (catalog# SPR-488), Pre-formed Fibrils (catalog# SPR-487) and Monomers (catalog# SPR-485) were utilized in SDS-PAGE/Western Blot (WB) and immunohistochemistry on human-derived AD brain sections. Aducanumab, known to have a preference for aggregated forms of Aβ, demonstrated binding to fibrillar amyloid beta in WB but not to monomeric or oligomeric amyloid beta.
Pre-adsorption of Aducanumab with Aβ pre-formed fibrils blocked antibody binding to Aβ deposits in AD brain tissue. Conversely, pre-adsorption with amyloid beta monomers did not block Aducanumab binding, clearly supporting Aducanumab’s specificity for fibrillar amyloid beta. Furthermore, Lecanemab, also known to favor aggregated Aβ, demonstrated a preference for fibrillar amyloid beta through WB analysis, albeit to a lesser extent than Aducanumab. However, immunohistochemical detection of Aβ deposits in an AD brain section were blocked by pre-adsorption with monomeric, oligomeric and fibrillar Aβ. As expected, Lecanemab was able to bind all amyloid beta variants.
By utilizing a Capillary Isoelectric Focusing (CIEF) assay with a panel of different length peptides, researchers were able to conclusively determine that Lecanemab recognizes a specific epitope found in the N-terminal region. Interestingly, Donanemab was found to have a preference for the Aβ 3-40 region bearing a pyroglutamate post-translational modification. As research into the complex mechanisms driving AD progression continues, pyroglutamate-modified amyloid beta has become a key variant of interest due to its increased aggregation propensity and enhanced neurotoxicity.
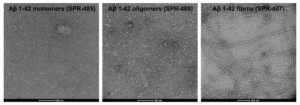
Figure 1: [Image from: StressMarq website] Transmission electron microscopy (TEM) of Amyloid Beta 1-42 Monomers (catalog# SPR-485), Amyloid Beta 1-42 Oligomers (catalog# SPR-488) and Amyloid Beta 1-42 PFFs (catalog# SPR-487), from left to right.
Summary
Using a combination of complementary biochemical assays, Talucci et al. were able to successfully epitope map a panel of 20 commonly used antibodies in Alzheimer’s disease research, including three commercially available therapeutic antibodies. This dataset will provide a valuable resource for researchers developing anti-amyloid beta antibodies targeted to critical variations influencing neurodegenerative disease pathology, such as mutations, truncations, post-translational modifications, or structural forms of the key proteins involved in these diseases, such as aggregated fibrils and oligomers. Access to such highly detailed antibody binding data for amyloid beta has the potential to accelerate Alzheimer’s disease research and facilitate the development of innovative therapeutic interventions.
Related StressMarq products
StressMarq specializes in the production of high-quality, cutting-edge tools for neurodegenerative disease research. With a broad range of amyloid beta reagents to meet all your research needs, including Amyloid Beta 3-42 Pyroglutamate Pre-formed Fibrils (catalog# SPR-492) and Recombinant Amyloid Beta 1-42 Oligomer Antibodies (catalog# SMC-618, SMC-619, SMC-620). Visit our website for more information, including the latest scientific publications using our specialized amyloid beta, alpha synuclein, and tau pre-formed fibrils, oligomers, and monomers.
References
- Epitope sequence and modification fingerprints of anti-Aβ antibodies. Talucci, I. et al., bioRxiv [Preprint]. 2025.


Leave a Reply