Advancing Neuroscience with StressMarq Antibodies
Protein misfolding in neurodegenerative diseases
Neurodegenerative diseases are debilitating conditions that affect millions of people worldwide. Progressive degeneration or death of nerve cells is responsible for symptoms like memory loss, anxiety and agitation. Disruption of the normal function of nerve cells is caused by misfolding and aggregation of proteins.
Antibodies detecting amyloid fibrils
Deposits of proteins in the brain are known as amyloid deposits. Amyloid deposits contain morphologically similar insoluble protein fibrils derived from different proteins, including alpha-synuclein, tau, and Aβ (amyloid beta) peptide.
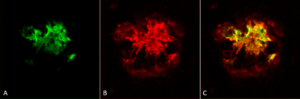
Figure 1. Immunohistochemistry analysis using Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (catalog# SPC-506).
StressMarq’s Anti-Amyloid Oligomers (A11) Antibody (catalog# SPC-506) can recognize all types of amyloid oligomers. This antibody was vital in a recent study that detailed the mechanism of neuronal release of alpha synuclein aggregates in amyloid deposits.
This study, published in Nature Communications, showed for the first time that in neurons, pathogenic alpha synuclein accumulates first in lysosomes. These toxic species are then released from the lysosomes into the extracellular space using a large protein family called SNARES1.
Antibodies detecting phosphorylated alpha synuclein
Alpha synuclein proteins aggregate in several neurodegenerative diseases, including Parkinson’s disease (PD), Lewy body dementia (LBD), and multiple system atrophy (MSA). Aggregates of alpha synuclein that cause disease are heavily phosphorylated at serine 129. In contrast, little phosphorylation occurs at this residue for physiological alpha synuclein.
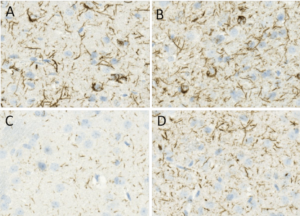
Figure 2. Immunohistochemistry analysis using Rabbit Anti-Alpha Synuclein pSer129 Monoclonal Antibody, Clone J18 (catalog# SMC-600).
Antibodies specific for phosphorylated alpha synuclein are essential tools for monitoring and quantifying alpha synuclein pathologies. Another recent study employed to describe pathways involved in the risk factor for PD, type-2 diabetes mellitus, and alpha synuclein pathogenesis2. Protein glycation, common in both type-2 diabetes mellitus and PD, is the chemical reaction between proteins and simple sugars or related structures.
Using mouse models, researchers showed that glycated alpha synuclein triggers PD-like features. StressMarq’s Anti-Alpha Synuclein Antibody was instrumental in demonstrating that glycation caused an increase in phosphorylated alpha synuclein in the mid-brain, aggravating PD-like features in mice. The study opens up the possibility of using anti-diabetic agents to affect glycation as a treatment for PD.
StressMarq products accelerate research into neurodegenerative disease processes
Antibodies that detect the presence of misfolded proteins or disease-causing forms of proteins are vital in neurodegenerative disease research. StressMarq produces high-quality antibodies that are systematically employed for this purpose and suitable for various experimental applications.
Specializing in research tools for studying neurodegenerative diseases, StressMarq also offers a range of neuroprotein constructs including monomeric, fibrillar and oligomeric Alpha Synuclein, Tau and Amyloid beta. Additional antibodies against alpha-synuclein and amyloid oligomers are also available, and are compatible with several applications, including immunohistochemistry, dot blot and western blot analysis.
References
- Lysosomal Exocytosis Releases Pathogenic α-Synuclein Species from Neurons. Xue Y. et al., BioRxiv 2021 April(10)439303.
- Glycation modulates glutamatergic signaling and exacerbates Parkinson’s disease-like phenotypes. Chegão, A. et al., Npj Parkinson’s Disease, 2022 8(1).


Leave a Reply