Bispecific Antibodies for the Treatment of Synucleinopathies
Parkinson’s disease (PD) and multiple system atrophy (MSA) are progressive neurodegenerative disorders that manifest through a combination of motor and non-motor symptoms. Both conditions belong to a broader category of diseases known as synucleinopathies. These are characterized by the abnormal accumulation of alpha synuclein in neurons and glial cells.
Under pathological conditions, alpha synuclein monomers misfold and aggregate into neurotoxic oligomers, protofibrils, and fibrils rich in β-sheet structures. These abnormal protein aggregates disrupt cellular homeostasis, leading to dysfunction and death of dopaminergic neurons. This compounding neuronal loss contributes to the hallmark symptoms of these neurodegenerative diseases, including bradykinesia, muscle rigidity and cognitive impairment.
Challenges in immunotherapy
The pathological spread of alpha synuclein throughout the brain is thought to occur in a progressive manner, and is closely associated with the worsening of clinical symptoms. One promising therapeutic strategy is immunotherapy, particularly the use of monoclonal antibodies such as Prasinezumab, which are currently in clinical development. These antibodies are designed to selectively bind to aggregated forms of alpha synuclein, potentially halting or slowing disease progression.
However, crossing the blood-brain barrier (BBB)—a highly selective semi-permeable membrane that restricts the passage of substances from the bloodstream into the brain—presents a major challenge in the effective delivery of therapeutic treatments. This limited permeability is primarily due to the tight junctions between the endothelial cells that form the BBB. Large molecules such as antibodies face significant difficulty in accessing the brain, with studies showing that only about 0.1–0.5% of an administered antibody dose is able to successfully cross the blood-brain barrier.
Traversing the blood-brain barrier
A multicenter, collaborative study conducted by researchers from ABL Bio, Sanofi, the University of Southern California, the University of California San Diego, and the National Research Council Canada, aimed to improve immunotherapy delivery for synucleinopathies by targeting receptor-mediated transcytosis (RMT). By exploiting naturally occurring vesicular transport mechanisms, An et al. sought to enhance the delivery of an engineered bispecific antibody that simultaneously targets alpha synuclein aggregates and the insulin-like growth factor 1 (IGF1R), a key mediator of RMT.
The bispecific antibody, referred to as SAR446159, not only demonstrated specific binding to alpha synuclein aggregates but also significantly reduced motor symptoms and the propagation of alpha synuclein pathology in established Parkinson’s disease models. Furthermore, compared to monospecific antibody treatments, SAR446159 exhibited improved penetration into both the brain and cerebrospinal fluid (CSF). These findings highlight the effectiveness of targeting IGF1R-mediated RMT to enhance the delivery and therapeutic impact of alpha synuclein antibody-based immunotherapy.
Preferential binding to fibrillar alpha synuclein
To initiate the study, it was essential to confirm that the bispecific alpha synuclein antibody selectively targeted aggregated forms of alpha synuclein, without binding to the corresponding monomeric units. Accordingly, researchers employed surface plasmon resonance (SPR) techniques to analyze molecular interactions between the antibodies and various conformations of alpha synuclein in real time.
The monospecific and bispecific SAR446159 antibodies were immobilized onto a sensor chip, and binding interactions were analyzed by injecting various concentrations of StressMarq’s Alpha Synuclein Monomers (catalog# SPR-321) and Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322) through the flow cell under continuous aqueous flow. Both the bispecific SAR446159 antibody and its monospecific counterpart exhibited a strong preference for binding to fibrillar alpha synuclein. Interestingly, both antibodies demonstrated a five-order-of-magnitude increase in affinity for fibrillar alpha synuclein, confirming their high selectivity for the aggregated, pathogenic form of the protein.
Structural variations in synucleinopathies
In addition to the different possible conformations of alpha synuclein, structural variations also exist between aggregates present in different synucleinopathies. Cryo-electron microscopy (Cryo-EM) studies have revealed that alpha synuclein fibrils from patients with PD and MSA can differ significantly in structure. Therefore, it was important to determine whether the bispecific SAR446159 antibody could recognize fibrillar alpha synuclein across various synucleinopathies.
Consequently, alpha synuclein aggregates were examined in post-mortem brain tissues from patients diagnosed with PD and MSA. For these immunostaining experiments, the monospecific alpha synuclein antibody was employed, as both the bispecific and monospecific antibodies share the same alpha synuclein-targeting component.
Positive immunostaining of alpha synuclein aggregates was observed in brain tissues representative of both synucleinopathies. Furthermore, disease pathology was detected in multiple brain regions, including the cerebral cortex and the substantia nigra pars compacta (SNpc), indicating that the antibody possesses a broad reactivity across different synucleinopathy-related aggregate structures.
Assessing neuroprotective potential
Misfolded and aggregated forms of alpha synuclein are transmitted from one neuron to another, in a prion-like manner. This behavior allows pathogenic alpha synuclein to enter neighboring cells and induce the misfolding of physiological, monomeric alpha synuclein. In Parkinson’s disease, this process is particularly detrimental to dopaminergic neurons, whose progressive degeneration results in symptoms such as bradykinesia and muscle rigidity.
To investigate the neuroprotective potential of SAR446159, researchers utilized co-culture models of human induced pluripotent stem cell (iPSC)-derived dopaminergic neurons and microglia, which mimic the complex brain microenvironment. Without antibody treatment, fluorescently-labeled pathogenic alpha synuclein was readily taken up by dopaminergic neurons. However, treatment with the bispecific SAR446159 antibody significantly reduced this uptake, suggesting a protective effect against the internalization of toxic aggregates.
Mitigation of cytotoxicity
Further experiments evaluated whether SAR446159 could also mitigate neuronal cell death in primary cortical neurons, which retain native neuronal properties without the need for differentiation. Exposure to StressMarq’s Alpha Synuclein A53T Mutant Pre-formed Fibrils (catalog# SPR-326)—which carry a mutation associated with early-onset Parkinson’s disease—induced a significant increase in cytotoxicity. Notably, this PFF-induced neuronal toxicity was significantly reduced following treatment with the bispecific SAR446159 antibody, highlighting its potential neuroprotective role.
In the concluding stage of the study, preclinical data gathered from in vivo Parkinson’s disease models indicated that treatment with the bispecific alpha synuclein SAR446159 antibody effectively reduced the severity of several motor symptoms. Furthermore, the spread of alpha synuclein was diminished with the bispecific treatment. Finally, targeting the IGF1R receptor resulted in notably increased penetration into the brain and CSF over a sustained period, when compared to the monospecific antibody.
Evaluating alpha synuclein bispecific therapies using StressMarq products
The intensive study by An et al. focused on addressing the major challenge of effectively delivering immunotherapeutic treatments to the brain through the blood-brain barrier. By targeting IGF1R, the bispecific alpha synuclein SAR446159 antibody was effectively transported across the BBB, making it a promising potential immunotherapeutic for treating synucleinopathies. In this study, SAR446159 was able to selectively bind StressMarq’s Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322), without binding the corresponding Alpha Synuclein Monomers (catalog# SPR-321). Furthermore, the neuroprotective role of the bispecific alpha synuclein SAR446159 antibody was examined in cellular assays following induction of neurotoxicity using StressMarq’s Alpha Synuclein A53T Mutant Pre-formed Fibrils (catalog# SPR-326).
Preclinical data indicates that the SAR446159 antibody has a strong preference for binding to alpha synuclein aggregates rather than monomers. Additionally, this bispecific antibody demonstrates decidedly improved penetration into the brain compared to its monospecific counterpart. An ongoing preclinical trial for bispecific SAR446159 will seek to further evaluate its potential to improve the treatment of synucleinopathies by enhancing the delivery of targeted antibody therapeutics.
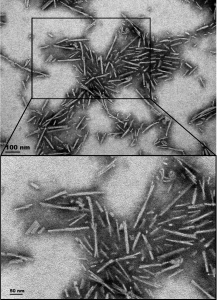
TEM of Human Alpha Synuclein A53T Mutant PFFs (catalog# SPR-326).
Related StressMarq products
StressMarq recognizes the complexities of protein structure and conformation in neurodegenerative diseases, and is dedicated to the development of innovative solutions to support neurodegenerative disease research. We offer a range of oligomeric, fibrillar and monomeric protein preparations of neurodegenerative proteins, including alpha synuclein. StressMarq’s fluorescently-tagged Alpha Synuclein Pre-formed Fibrils: ATTO 594 (catalog# SPR-322-A594) can be utilized to visualize the induction of pathological aggregation. Visit our website for more information, including the latest scientific publications using our specialized alpha synuclein, tau and amyloid beta protein constructs.
References
- A brain-shuttled antibody targeting alpha synuclein aggregates for the treatment of synucleinopathies. An, S. et al. bioRxiv. 2025. Please note that this article is a preprint and has not been certified by peer review.
- Structures of α-synuclein filaments from human brains with Lewy pathology. Yang, Y. et al. Nature. 2022.

Leave a Reply