EGCG Alpha Synuclein Oligomers
Alpha Synuclein (α-syn) is a 14 kDa synaptic protein known for its association with neurodegenerative disorders such as Parkinson’s disease. Mutations in alpha synuclein linked to Parkinson’s disease promote aggregation of soluble alpha synuclein monomers into oligomer intermediates, then further aggregation results in the formation of insoluble fibrils displaying a beta-sheet structure. It is these insoluble fibrils which are the main components of Lewy bodies observed in patients suffering from Parkinson’s disease. However, it is the oligomer intermediates which are thought to exert the most toxic effects on neuronal cells. The oligomers are able to seed and spread through the nervous system in a prion-like manner, from affected neurons to unaffected neurons (1, 2).
The flavonoid epigallocatechin gallate (EGCG) found in green tea, has been shown in studies to stabilize alpha synuclein in its oligomeric form. Interestingly, EGCG promotes the formation of non-toxic oligomers, and it has been reported that immunization with recombinant alpha synuclein can improve symptoms in a transgenic mouse model of Parkinson’s disease (3). These soluble non-toxic oligomers, reported to be spherical and generally unstructured, are also unable to seed alpha synuclein monomers (4).
How does EGCG stabilize the oligomeric form of alpha synuclein?
Research suggests that the oligomers are stabilized by EGCG in two ways, firstly by blocking aggregation of monomers into fibrils and secondly by disassembling mature fibrils, remodeling their structure. Using a combination of microscopy and spectroscopy, EGCG was shown to directly bind assembled alpha synuclein fibrils, resulting in a remodeling of their beta-sheet structure into smaller amorphous protein aggregates. This remodeled protein lost its ability to seed aggregation of alpha synuclein monomers and displayed reduced cellular toxicity (5).
In a 2016 study by Xu et al, the presence of EGCG was shown to block alpha synuclein aggregation into fibrils in a concentration dependent manner. EGCG inhibition of alpha synuclein fibril formation was demonstrated in three different experiments; a thioflavin T binding assay using recombinant human alpha synuclein, fluorescence-labelled alpha synuclein binding to fibrils coated on microplates, and fluorescence-labelled alpha synuclein binding to aggregated alpha synuclein in post-mortem Parkinson’s disease tissue (6). Research by Zhao et al, looked further into the interaction between EGCG and alpha synuclein using 1H NMR. Results indicated remodeling of mature fibrils may be due to the interaction of EGCG with the residues of Leu, His, Phe and Tyr of α-syn. Whereas blocking of the aggregation of unstructured monomers into beta-sheet rich fibrils may be due to the interaction of EGCG with the residues of Ile, Phe and Tyr on the monomer (7).
How does EGCG affect oligomer toxicity?
It is thought that EGCG-stabilized oligomers are non-toxic due to an inability to permeabilize membranes. Lorenzen et al in their 2014 publication suggest that a reduction in oligomer toxicity is due to immobilization of the alpha synuclein C-terminal lipid-binding region on EGCG binding. This results in the oligomers having a reduced ability to interact with membranes. It is this membrane binding which leads to cell permeabilization, a known toxic effect of alpha synuclein oligomers. Surprisingly, this group did not find the structural changes on EGCG binding described in other studies (8). Though the mechanism by which EGCG exerts it effects is not yet clear, as a small molecule that can pass through the blood-brain barrier, EGCG is a potential agent for the treatment of Parkinson’s disease.
Now available from StressMarq our highly active alpha synuclein oligomers were developed for research into Parkinson’s disease and other α-synucleinopathies. Our range includes human recombinant alpha synuclein oligomers (Epigallocatechin Gallate Stabilized), SPR-469, shown not to seed alpha synuclein monomer (SPR-321) in Thioflavin T assays.
For more information and details of additional alpha synuclein products in our range, please visit our website.
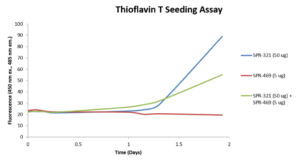
Thioflavin T assay of EGCG-stabilized alpha synuclein oligomers (SPR-469) and alpha synuclein monomers (SPR-321). The oligomers do not seed aggregation of monomers and may have an inhibitory effect.
REFERENCES
1) Ingelsson, M. Front Neurosci. 2016; 10:408.
2) Rey, N.L. et al. Acta Neuropathol. 2013; 126:555–573.
3) Bengoa-Vergniory, N. et al. Acta Neuropathol. 2017; 134(6):819-838.
4) Ehrnhoefer, D.E. et al. Nat Struct Mol Biol. 2008; 15(6):558-566.
5) Bieschke, J. et al. PNAS 2010; 107 (17) 7710-7715.
6) Xu, Y. et al. Neurochem Res. 2016; 41(10):2788-2796.
7) Zhao, J. et al. RSC Adv. 2017; 7, 32508-32517.
8) Lorenzen, N. et al. JBC. 2014; 289, 21299-21310.


Leave a Reply