Oxidative DNA Damage and its Role in Disease
Cellular DNA is an intrinsically reactive biomolecule that is continuously exposed to agents with the potential to cause harm. Included among these, reactive oxygen species (ROS) generated as by-products of aerobic respiration and metabolism give rise to background levels of DNA damage that are increased when ROS are present in excess1. In response to oxidative DNA damage, cells activate complex signaling networks to promote DNA repair and survival or trigger cell death2. However, when these mechanisms fail, genomic instability can lead to disease. Understanding the pathological role of oxidative DNA damage is critical to develop effective therapies for conditions including cancer, Alzheimer’s disease, and diabetes.
What are some types of DNA damage?
DNA damage can broadly be divided into that caused by exogenous factors and that which occurs endogenously. Examples of exogenous DNA damaging agents include UV radiation that causes covalent linkages to form between adjacent pyrimidines; ionizing radiation (IR) that generates base lesions and single strand breaks; and chemicals present in tobacco smoke that are converted into alkylating agents able to induce mispairing between bases. Causes of endogenous DNA damage include replication errors such as base substitutions or single base insertions; spontaneous base deamination that results in base conversions; and oxidative stress, whereby ROS that are naturally present in cells damage DNA via several different mechanisms1.
How is DNA damaged by oxidative stress?
Under normal conditions, ROS are essential to cell growth and survival. For example, by interacting with critical signaling molecules, ROS drive processes including proliferation, apoptosis, and iron homeostasis, while their release from macrophages and neutrophils represents an important defense mechanism against invading pathogens3,4. Yet, under conditions of oxidative stress (an imbalance between ROS production and an organism’s antioxidant defenses), excess ROS levels have been shown to cause approximately 100 different oxidative base lesions and 2-deoxyribose modifications1. Of the most widely studied types of ROS – namely, superoxide radicals (•O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (•OH) – the hydroxyl radical is by far the most reactive1. Produced during the reaction between H2O2 and Fe2+, the hydroxyl radical causes DNA damage through its addition to the double bonds of DNA bases and its abstraction of hydrogen atoms from methyl groups and the C-H bonds of 2′-deoxyribose sugars5.
What diseases arise from oxidative DNA damage?
Oxidative DNA damage has been linked to a broad range of diseases and is widely recognized for its contributory role in the initiation and progression of various types of cancer5,6. Notably, 8-hydroxy-2′-deoxyguanosine (8-OHdG) is a predominant oxidative base lesion that is used as a biomarker of oxidative stress and cancer, and is frequently used to estimate the extent of DNA damage in patients following exposure to carcinogens such as tobacco smoke, asbestos fibers, or heavy metals7. Other conditions associated with oxidative DNA damage include neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease; autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus (SLE); as well as diabetes, cardiovascular disease, and cystic fibrosis.
Advancing understanding of oxidative DNA damage
StressMarq Biosciences offers a broad portfolio of reagents for studying oxidative DNA damage and its role in disease pathogenesis. These include our Anti-DNA/RNA Damage Antibody [15A3] (SMC-155) that is validated for multiple applications and our DNA Damage (8-OHdG) ELISA kit (SKT-120) for colorimetric detection of the 8-OHdg biomarker in sample material such as plasma or urine.
Visit our website to use our interactive graph to explore diseases related to oxidative stress, and to see a list of all of our products for studying Oxidative Stress.
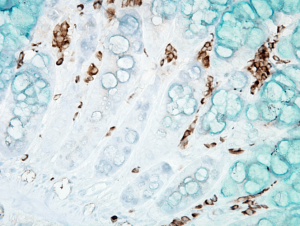
IHC analysis using Mouse Anti-DNA Damage Monoclonal Antibody, Clone 15A3 (SMC-155). Tissue: inflamed colon. Species: Mouse.
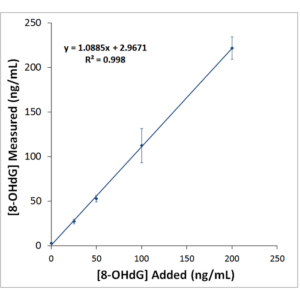
Typical Standard Curve for the DNA Damage (8-OHdG) ELISA kit (SKT-120). Competitive ELISA format, assay range: 0.94 – 60 ng/ml.
References
- Mechanisms of DNA damage, repair and mutagenesis, Chatterjee N and Walker GC, Environ Mol Mutagen. 2017 Jun;58(5):235-263
- DNA damage and the balance between survival and death in cancer biology, Roos WP et al, Nat Rev Cancer. 2016 16, 20–33
- Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling, Ray PD et al, Cell Signal. 2012 May;24(5):981-90
- Mitochondrial reactive oxygen species as major effectors of antimicrobial immunity, Shekhova E, PLoS Pathog 2020 16(5)
- Oxidative DNA damage: mechanisms, mutation, and disease, Cooke MS et al, FASEB J. 2003 Jul;17(10):1195-214
- Oxidative Stress and Cancer, Klaunig JE, Curr Pharm Des. 2018;24(40):4771-4778
- 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis, Valavanidis A et al, J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009 Apr;27(2):120-39


Leave a Reply