PCT Patent Application: Monoclonal Antibody Targets Alpha Synuclein and Tau Aggregation
StressMarq has recently had its PCT patent application describing a novel therapeutic antibody candidate published. This antibody binds both alpha synuclein and tau, two proteins that aggregate into toxic fibrils in neurodegenerative diseases such as Parkinson’s and Alzheimer’s. This potential therapeutic monoclonal antibody (Clone 2F11), still at the pre-clinical stage of development, moves the company into the drug discovery field and is unique in that it could potentially neutralize multiple disease targets.
Alzheimer’s and Parkinson’s diseases are the two most prevalent neurodegenerative diseases and are poised to become more prevalent as populations age. Parkinson’s Disease (PD) is characterized by the aggregation of a 14 kDa protein called alpha synuclein. Alpha synuclein monomers aggregate to form oligomers, fibrils, and ultimately deposits known as Lewy bodies. This aggregation leads to neuronal death.1 Alpha synuclein fibrils and oligomers exist in a dynamic equilibrium where fibrils can disassemble into oligomers and oligomers can aggregate to form fibrils.2 Alpha synuclein oligomers are believed to have prion-like properties; they can spread between cells and recruit monomers to form fibrils.3,4 Not all oligomers are pathogenic; some are considered “off-pathway” to fibrillization.5 The structure of an oligomer determines whether it promotes or inhibits fibrillization.6
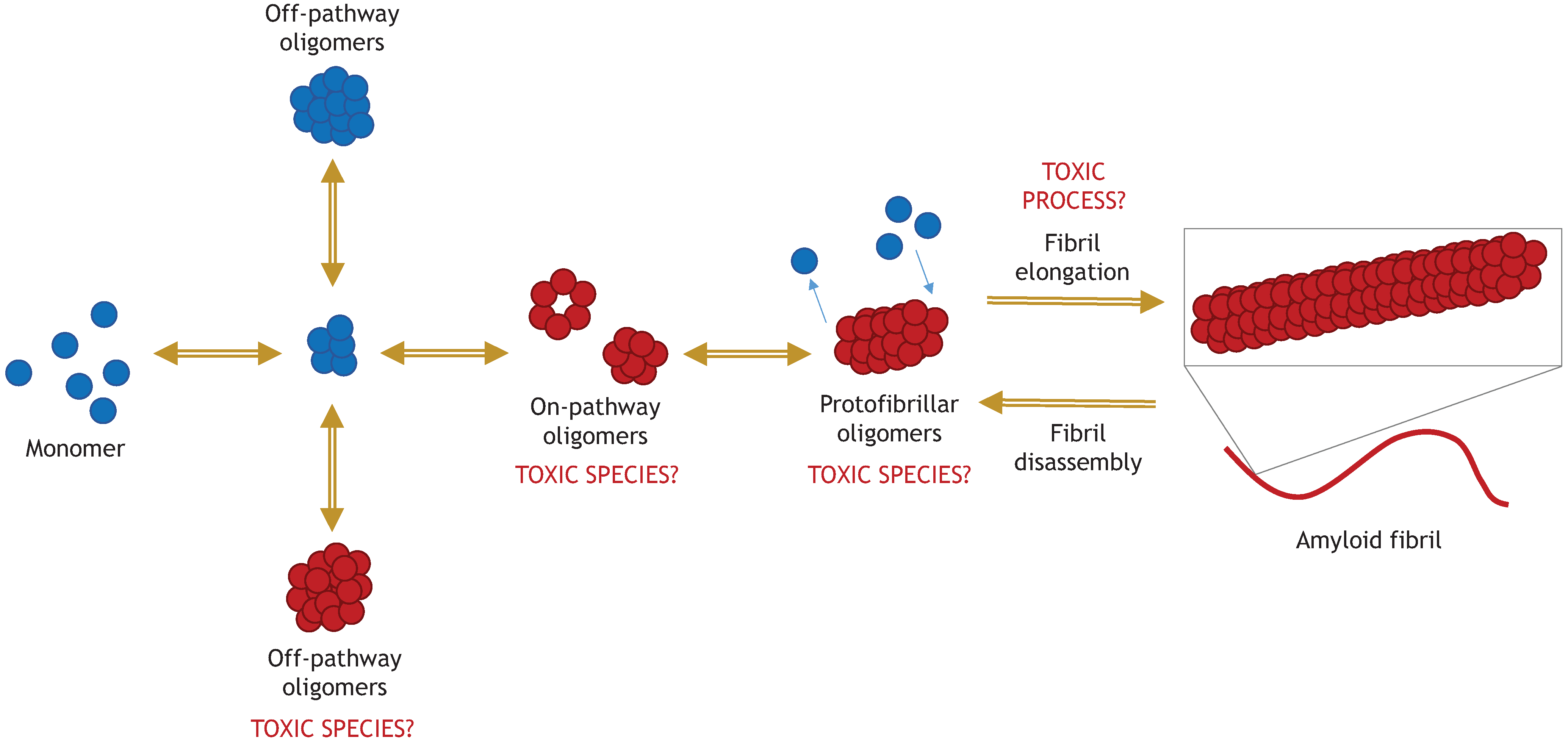
Toxic α-synuclein oligomers in relation to the pathway of amyloid fibril formation.5 Roberts, H. and Brown, D. R. Biomolecules 2015, 5(2), 282-305; doi:10.3390/biom5020282
While other antibodies have been generated against alpha synuclein, this one is unique in that it binds to pathogenic alpha synuclein. StressMarq specializes in manufacturing research tools for Parkinson’s Disease including alpha synuclein preformed fibrils (PFFs) that induce aggregation and Lewy body pathology in vitro and in vivo. This antibody has been shown to neutralize alpha synuclein aggregation in vitro and in primary cells that were seeded with pathogenic alpha synuclein PFFs. This positions it as a potential therapeutic antibody for Parkinson’s Disease. Antibodies have recently gained significant interest as therapeutic agents due to their high specificity and low toxicity profiles, differentiating them from most other drug candidates such as small molecules.
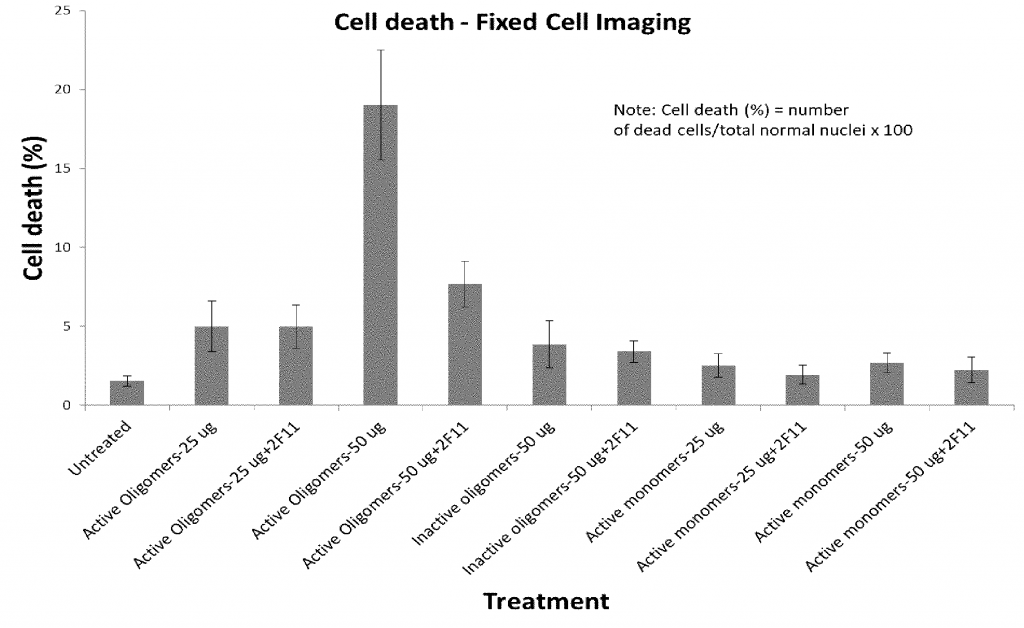
Figure shows cell death of human SHSY-5Y cells in the presence of active α-synuclein oligomers, inactive α-synuclein oligomers, active α-synuclein monomers in the presence and absence of the 2F11 antibody. Cell death (%): Number of dead cells/Total normal nuclei x 100. Adding 2F11 antibody SHSY-5Y cells with active oligomers significantly reduced cell death.
Early stage data also suggests an ability to significantly reduce tau aggregation, a therapeutic target in Alzheimer’s Disease (AD). AD is characterized by plaques consisting of amyloid-beta and neurofibrillary tangles (NFTs) consisting of tau. Therapeutic approaches targeting amyloid-beta have shown little success, so tau is becoming an increasingly attractive target. Traditionally, Lewy bodies have been associated with PD and NFTs with AD, but there is significant overlap and co-occurrence of alpha synuclein and tau pathologies in a spectrum of neurodegenerative diseases.7
This antibody recognizes the 3D protein structure of its targets, opening up the possibility that it could treat two or more prevalent neurological diseases with similar protein fibril structures.
See related press release and patent application.
References
- Osterberg, V. R. et al. Progressive aggregation of alpha-synuclein and selective degeneration of lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell Rep. 10, 1252–60 (2015).
- Cremades, N. et al. Direct observation of the interconversion of normal and toxic forms of α-synuclein. Cell. 149(5):1048-59 (2012).
- Bartels, T. et al. α-synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 477(7362):107-10 (2011).
- Wang, W. et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 108(43):17797-802 (2011).
- Roberts, H. L., Brown, D. R. Seeking a mechanism for the toxicity of oligomeric α-synuclein. Biomolecules. 5(2):282-305 (2015).
- Horvath, I. et al. Modulation of α-synuclein fibrillization by ring-fused 2-pyridones: templation and inhibition involve oligomers with different structure. Arch Biochem Biophys. 532(2):84-90 (2013).
- Li, X. et al. Interactions Between α-Synuclein and Tau Protein: Implications to Neurodegenerative Disorders. J Mol Neurosci. 60(3):398-304 (2016).

Leave a Reply