Shining Light on the Cell-to-Cell Spread of Alpha-Synuclein
Synucleinopathies are neurodegenerative disorders characterized by abnormal deposits of proteins inside brain cells known as Lewy bodies (LB). Examples include Parkinson’s disease (PD) and dementia with Lewy bodies (DLB). Patients with synucleinopathies have coordination difficulties and cognitive impairment, depending on the distribution pattern of LBs. A major component of LBs is alpha synuclein protein. Alpha synuclein is a 140-amino-acid protein abundantly expressed in the nervous system, predominately in neurons and presynaptic vessels. Monomeric forms of alpha synuclein can self-assemble to form oligomers and then aggregate to form fibrillary forms (pre-formed fibrils or PFFs). Oligomers and aggregates of alpha synuclein are neurotoxic and can spread to different parts of the brain, which contributes to the pathogenesis of PD and DLB. Also, alpha synuclein PFFs act as “seeds” to increase the recruitment of alpha synuclein monomers into fibrils. A recent study by Chen et al., identifies neuronal receptor low-density lipoprotein receptor-related protein 1 (LRP1) as a potential therapeutic target for synucleinopathies due to its regulation of alpha synuclein uptake and spread between neurons [1].
LRP1 receptor regulates the cell-to-cell transmission of alpha-synuclein
Chen and co-authors chose to study the LRP1 because this transmembrane receptor has important functions in regulating the endocytosis of many ligands, including tau. Tau is a microtubule-associated protein that self-assembles abnormally to form insoluble tangles in Alzheimer’s disease. LRP1 has been shown to be involved in the uptake and spread of tau between neurons to different parts of the brain.
Chen et al., investigated whether LRP1 plays a role in alpha synuclein uptake. Neuronal stem cells were used as a cell model, and the importance of LRP1 was investigated in these cells by knocking out LRP1 with the CRISPR/Cas9 technique. Alpha synuclein uptake was investigated by fluorescently labelling monomeric alpha synuclein and measuring protein uptake via endocytosis using flow cytometry.
In neuronal cells, LRP1-deficiency reduced endocytosis of monomeric forms of alpha synuclein by more than 75% compared to control cells. A known LRP1 binding antagonist, RAP, could also compete with alpha synuclein in a dose-dependent manner for neuronal uptake by LRP1. Therefore, the study authors demonstrated that the LRP1 receptor may be a key endocytic receptor for not only tau but also monomeric alpha synuclein in neurons.
LRP1 regulates the uptake of oligomers and aggregated forms of alpha-synuclein
As oligomers and aggregated forms of alpha-synuclein are neurotoxic, the authors then tried to understand whether LRP1 could regulate these forms of alpha synuclein. For this, the authors used two products from StressMarq: Kinetically Stable Alpha Synuclein Oligomers (catalog# SPR-484) and Type 1 Alpha Synuclein Pre-formed Fibrils (PFFs) (catalog# SPR-322).
The study authors confirmed the structure of the alpha synuclein oligomers and PFFs using negative stain transmission electron microscopy (TEM). Protein uptake assays assessed internalization via endocytosis of fluorescently labelled forms of alpha synuclein. When LRP1 was removed from neurons, the protein uptake of alpha-synuclein oligomers halved compared to control cells. For alpha synuclein PFFs, removal of the LRP1 receptor only reduced alpha-synuclein uptake by 5–10%. The study suggests that LRP1 is a key regulator for the endocytosis of soluble forms of alpha-synuclein (monomers and oligomers). On the other hand, aggregated forms are less dependent on LRP1 in neurons and may involve multiple or different mechanisms for internalization.
Next, the researchers studied which part of alpha synuclein was implicated in the LRP1-mediated uptake of alpha-synuclein. Interestingly, alpha synuclein is similar to tau in that it has a high content of lysines (10%). The authors showed that, like tau, LRP1 likely also interacts with the lysine-rich N-terminus of alpha-synuclein through lysine residues.
Mouse models show that LRP1 regulates the spread of alpha-synuclein in the brain
To study the biological importance of LRP1 on the spread of alpha-synuclein in neurons, a conditional transgenic mouse model that deleted the Lrp1 gene in neurons was used. By analysing the brain tissue from Lrp1 knockout mice and control mice, the authors showed that Lrp1 deletion suppresses the spread of alpha-synuclein in mouse brains.
In summary, Chen and co-authors have demonstrated evidence that LRP1 regulates endocytosis and uptake of alpha-synuclein, and deletion of the LRP1 neuronal receptor can reduce the spread of alpha-synuclein in the mouse brain. This opens the possibility of developing therapeutic agents that disrupt the monomeric and oligomeric uptake of alpha-synuclein via LRP1, which could reduce the spread and pathogenesis of alpha-synuclein in neuronal pathologies such as PD and DLB.
StressMarq’s products support research into the cell-to-cell transmission of alpha-synuclein
StressMarq’s wide variety of alpha synuclein monomers, oligomers, and pre-formed fibrils provide tools vital for studying the cell-to-cell transmission of alpha-synuclein in disease models such as PD and DLB. In the study by Chen et al. the LRP1 receptor was identified to regulate the uptake of oligomeric alpha-synuclein. In contrast, protein internalization of aggregated alpha-synuclein PFF may be mediated by different or multiple mechanisms. Alpha-synuclein products from StressMarq undergo extensive structural characterization (Figure 1).
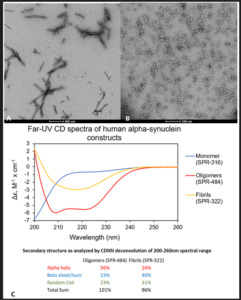
Figure 1. Alpha Synuclein PFFs and Oligomers from StressMarq are key research tools for alpha synuclein. Transmission electron microscopy (TEM) of Alpha Synuclein PFFs (SPR-322) (A) and Alpha Synuclein Oligomers (SPR-484) (B). Αlpha Synuclein Oligomers have distinct secondary structure differences compared to fibrils (C).
UV-CD data suggests that StressMarq’s Alpha Synuclein Oligomers have distinct secondary structure differences compared to monomers and fibrils. More specifically, StressMarq’s Kinetically Stable Alpha Synuclein Oligomers (catalog#SPR-484) show a significantly higher alpha helix content and lower beta-sheet/turn content than Alpha Synuclein Pre-formed Fibrils (Type 1) (catalog#SPR-322). StressMarq’s Alpha Synuclein Monomers (catalog#SPR-316) show a strong negative signal at 200 nm indicative of a disordered protein state (low secondary structure content).
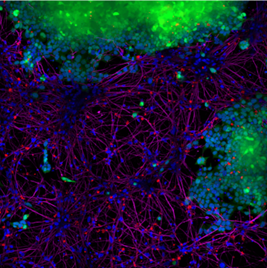
In a different experiment, StressMarq’s Alpha Synuclein PFFs (red) catalog#SPR-322, were shown to be taken up by SH-SY5Y cells and transmitted to neuronal iPSCs within 14 days. Blue: Hoechst/DNA; Green: SHSY5Y-GFP; Red: alpha-synuclein PFFs-555 (cat#SPR-322); Purple: Tubulin.
StressMarq also sells a wide range of antibodies for neuroscience research, including two polyclonal antibodies for LRP-1, catalog# SPC-784 which can be used for Western blotting, and catalog# SPC-785 which can be used for Western blotting and immunohistochemistry (IHC).
References
[1] K. Chen et al., ‘LRP1 is a neuronal receptor for α-synuclein uptake and spread’, Mol Neurodegener, vol. 17, no. 1, p. 57, 2022, doi: 10.1186/s13024-022-00560-w.


Leave a Reply