The Role of VMP1 in Regulating NLRP3 Inflammasome Responses
Inflammation is a vital cellular process that controls infection. The inflammasome, a multiprotein complex, assembles in response to foreign pathogens or tissue damage, causing activation of caspase-1 and inducing inflammation. This process must be highly regulated to avoid dysregulated or excessive inflammatory responses associated with neurodegenerative diseases like Parkinson’s disease (PD).
Vacuole Membrane Protein 1 (VMP1), a protein involved in membrane trafficking, has been implicated in multiple diseases and cellular processes, including pancreatic cancer, SARS-CoV-2 infection, and impaired mitochondrial homeostasis. In PD patients, VMP1 expression is decreased in peripheral blood mononuclear cells (PBMCs)1. A new study by Zack et al., elucidates the cellular mechanisms by which VMP1 depletion can augment inflammasome activation and consequently enhance inflammation. This study adds to the growing body of evidence supporting diagnostic and therapeutic applications that target VMP1, particularly for neurodegenerative diseases like PD.
VMP1 depletion increases NLRP3 inflammasome activation
The study employed a commonly used cellular model for macrophages and monocytes, THP-1 cells, to study the effect of VMP1 on the inflammasome. Efficient CRISPR-based depletion of VMP1 was verified in these cells by assessing endogenous VMP1 protein levels using an anti-VMP1 antibody from StressMarq Biosciences (catalog# SPC-680) in Western blot analysis. Depletion of VMP1 resulted in the release of pro-inflammatory molecules at both basal levels and upon stimulation of inflammatory pathways.
The authors then questioned whether the activation of the NLRP3 inflammasome caused the secretion of pro-inflammatory cytokines in VMP1-depleted cells. By treating primed VMP1 KO macrophages with α-synuclein fibrils, activation of the NLRP3 inflammasome was demonstrated to be upregulated compared to control cells. RNA sequencing experiments were then performed to understand how VMP1 deregulation could control the NLRP3 inflammasome. Depletion of VMP1 led to the downregulation of a calcium ATPase pump, sarcoplasmic/ER Ca2+-ATPase (SERCA), and an upregulation of genes associated with calcium signaling and alterations in autophagy genes. SERCA is crucial for calcium-dependent homeostasis.
VMP1 depletion causes mitochondrial dysfunction and disrupts cellular homeostasis
Monitoring the intracellular calcium response to inflammatory pathway activators in live cell analysis confirmed greater intracellular calcium levels with VMP1 depletion. Chemical inhibition of SERCA suggested inhibition of these pumps plays a role in the augmented inflammasome activation seen in the context of VMP1 depletion. As mitochondria are regulators of cellular calcium, the involvement of these organelles was assessed.
In mitochondria, depletion of VMP1 resulted in calcium overload, mitochondrial dysfunction, and the consequent release of mitochondrial DNA into the cytoplasm. Given that VMP1 is an autophagy adaptor, the effects of VMP1 depletion were examined, and the results were indicative of impaired autophagic degradation activity. Alteration in autophagy activity is a likely consequence of the mitochondrial damage seen with VMP1 depletion.
StressMarq products for VMP1-related research
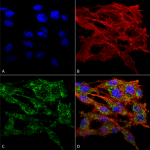
Pro-inflammatory cytokine secretion caused by the NLRP3 inflammasome is involved in the pathophysiology of neurodegeneration. As demonstrated by Zack et al., VMP1 can negatively regulate NLRP3 inflammasome responses by altering SERCA and autophagy activity, with the result being irregular intracellular calcium signaling and mitochondrial dysfunction. Taken together with other studies, the importance of VMP1 as an endogenous diagnostic marker for PD is becoming increasingly evident. StressMarq’s VMP1 antibody (catalog# SPC-680) was critical in characterizing endogenous VMP1 in a monocyte/macrophage cellular model used in the study. The antibody has been validated in Western blot/immunocytochemistry/immunofluorescence applications (Figure 1).
Figure 1. Immunocytochemistry/Immunofluorescence analysis using Rabbit Anti-VMP1 Polyclonal Antibody (catalog# SPC-680)
Related StressMarq products
StressMarq is a trusted manufacturer of a vast range of antibodies, proteins, and inhibitors for neuroinflammatory diseases. Like clinically-developed therapies, StressMarq’s NLRP3 inflammasome activation inhibitor (catalog# SIH-604) can be used to prevent an amplified inflammatory response by inhibiting the NLRP3 inflammasome. Lysosomes are involved in both inflammasome activation and autophagy.
Anti-LAMP1 antibody (catalog# SMC-140) from StressMarq immunolabels lysosome-associated membrane proteins, or LAMP1, a major constituent of the lysosomal membrane. Finally, Stressmarq offers convenient human alpha synuclein PFFs (catalog# SPR-322) to assist in characterizing NLRP3 inflammasome activation events.
References
- Abnormal Vacuole Membrane Protein-1 Expression in Parkinson’s Disease Patients. Al-Nusaif, M. et al. https://pubmed.ncbi.nlm.nih.gov/35464320/.
- Vacuole Membrane Protein 1 (VMP1) Restricts NLRP3 Inflammasome Activation by Modulating SERCA Activity and Autophagy. Zack, S. R. et al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9900977/. Note: This is a preprint. The article has not been peer-reviewed by a journal.


Leave a Reply