The Role of Wild Type SOD1 in ALS
The superoxide dismutase (SOD) family of enzymes functions to protect cells from reactive oxygen species, specifically by converting superoxide radicals (O2·-) to molecular oxygen (O2) and hydrogen peroxide (H2O2). It comprises SOD1, a copper and zinc-containing homodimer found almost exclusively in intracellular cytoplasmic spaces; SOD2, a manganese-containing tetramer located within mitochondria; and SOD3, a copper and zinc-containing tetramer found predominantly in the extracellular matrix1. SOD1, the first family member to be described, has been linked to amyotrophic lateral sclerosis (ALS), a severe motor neuron disease characterized by progressive degeneration of motor neurons in the brain and spinal cord. Currently, with no available cure, ALS culminates in paralysis and death within just 2 – 5 years of onset, highlighting the importance of SOD1 as a potential therapeutic target.
What is the role of WT SOD1 in ALS?
Around 90% of cases of ALS are sporadic (SALS), with the remaining 10% being familial (FALS)2. However, clinical similarities between both forms of the disease have long suggested them to share a common pathogenic pathway and/or involve similar toxic factors3,4. SOD1 is now known to be one such factor, following the identification in 1993 of 11 different SOD1 missense mutations in 13 different FALS families5. Since then, more than 150 SOD1 mutations have been discovered in ALS patients (including point mutations and protein truncations resulting from early termination), accounting for 20–25% of all FALS cases and a small percentage of SALS cases3,6. Critically, ALS-associated mutations in SOD1 induce protein misfolding and aggregation in axons to cause neuronal cell death. For this reason, SOD1 has become one of the most widely studied genes within the field of ALS research and is considered to be a promising target for therapeutic intervention.
Not only is mutant SOD1 thought to drive ALS onset and progression, but the wild-type (WT) SOD1 protein is also believed to be an important modulator of disease. Key findings to support this hypothesis include the observation that non-genetic disturbances such as metal depletion, oxidation, and disruption of the quaternary structure can induce WT SOD1 to misfold; the discovery that WT SOD1 immunopurified post-mortem from the spinal cord of a patient with SALS can inhibit kinesin-based fast axonal transport in a manner similar to a FALS-linked mutant; and the generation of data showing that co-expression of high levels of WT human SOD1 with various mutant forms (A4V, G85R, G93A, T116X, or L126Z) of the human protein in transgenic mice can accelerate the course of disease3,4,6. Additionally, ELISA-based evaluation of cerebrospinal fluid (CSF) from a cohort of patients with SALS found WT SOD1 to be misfolded in all of the cases examined; while the CSF samples exhibited significant toxicity toward motor neuron-like NSC-34 cells grown in culture, this effect could be ameliorated by removing the misfolded WT SOD1 protein via immunoprecipitation2.
Supporting the study of wild-type SOD1
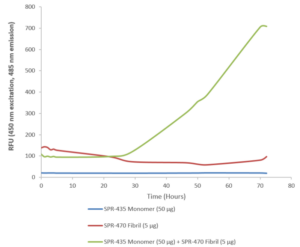
StressMarq offers a broad selection of products for researchers wishing to study wild-type SOD1. These include our human recombinant SOD1 protein monomer (SPR-435) and our human recombinant SOD1 protein preformed fibrils (SPR-470) that are readily combined in vitro to investigate SOD1 aggregation.
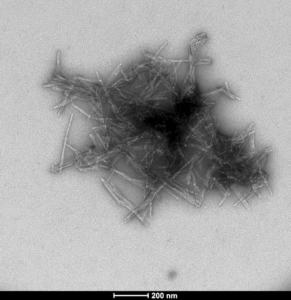
TEM of human SOD1 pre-formed fibrils (PFFs) (SPR-470)
Additional information on our SOD1 products, including Protocols, handling instructions and FAQs can be found on our website.
References
- Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression, Zelko IN et al, Free Radic Biol Med. 2002 Aug 1;33(3):337-49
- Wild-type Cu/Zn-superoxide dismutase is misfolded in cerebrospinal fluid of sporadic amyotrophic lateral sclerosis, Tokuda E et al, Mol Neurodegener. 2019 Nov 19;14(1):42
- An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis, Rotunno MS and Bosco DA, Front Cell Neurosci. 2013 Dec 16;7:253
- Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS, Bosco DA et al, Nat Neurosci. 2010 Nov;13(11):1396-403
- Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis, Rosen DR et al, Nature. 1993 Mar 4;362(6415):59-62
- An examination of wild-type SOD1 in modulating the toxicity and aggregation of ALS-associated mutant SOD1, Prudencio M et al, Hum Mol Genet. 2010 Dec 15;19(24):4774-89

Leave a Reply