Unraveling the Complexity of Tau Protein Isoforms
A Key to Understanding Neurodegenerative Diseases
Introduction
In the field of neuroscience, the study of tau protein has emerged as a pivotal area of research. Tau proteins are primarily expressed in neurons and play a vital role in stabilizing microtubules, which are crucial for maintaining the structural integrity and transport within neurons. However, abnormalities in tau proteins have been implicated in the development of neurodegenerative diseases, such as Alzheimer’s disease, frontotemporal dementia, and chronic traumatic encephalopathy.
The accumulation of tau protein aggregates into neurofibrillary tangles (NFTs) is a hallmark pathological feature of tauopathies, which are neurodegenerative disorders characterized by the deposition of abnormal tau protein in the brain. NFTs primarily consist of hyperphosphorylated tau protein isoforms, which disrupt the normal functioning of neurons and contribute to their degeneration.
Tau Isoforms
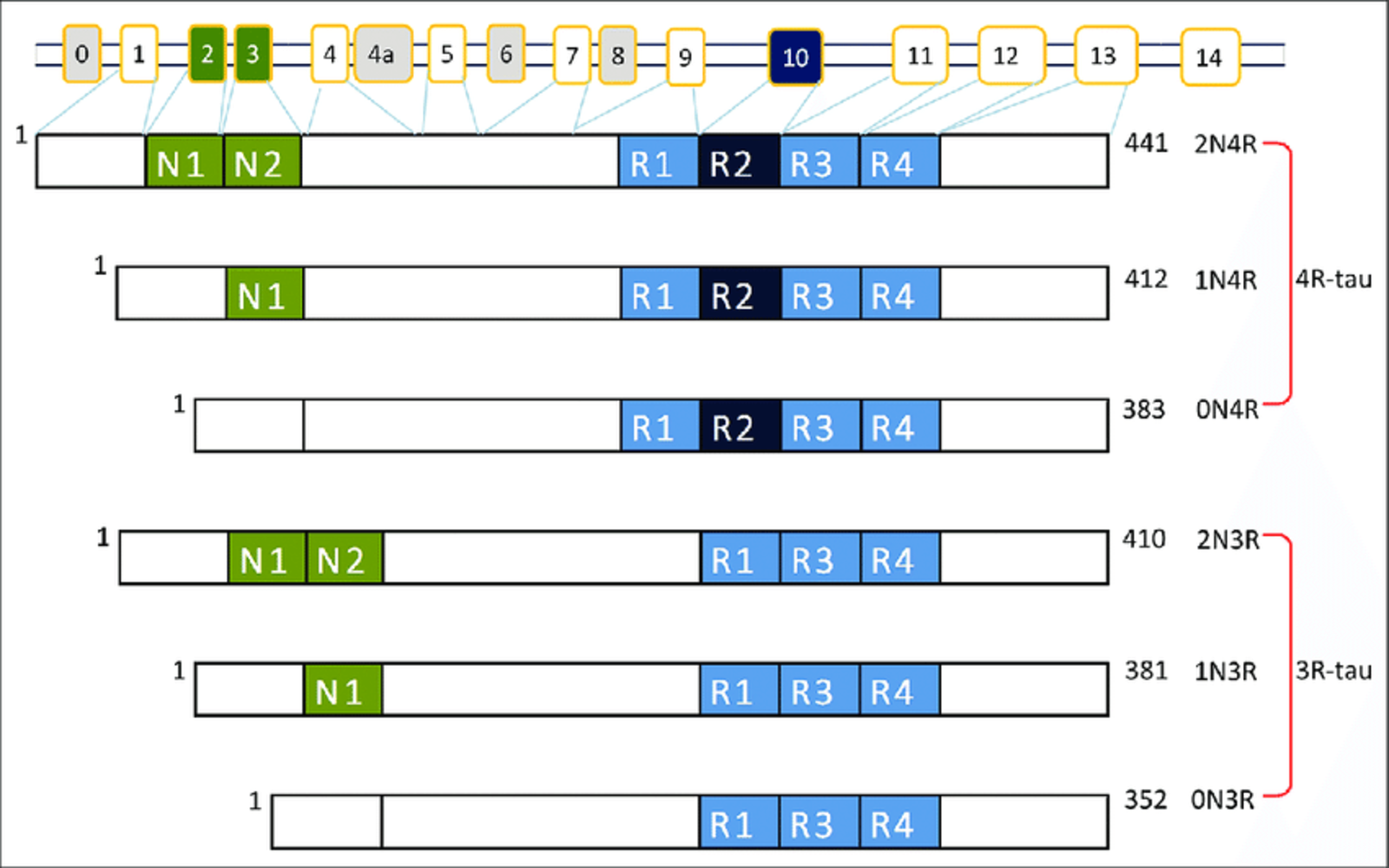
There are six different tau protein isoforms in humans as a result of the gene (MAPT) undergoing alternative splicing. These isoforms differ in the presence or absence of specific amino acid sequences, resulting in variations in protein length and charge.
The six tau protein isoforms are classified based on the presence or absence of exon 2, exon 3, and exon 10. The isoforms are named as 0N, 1N, or 2N (where “N” refers to the number of N-terminal inserts), and as 0R, 1R, or 2R (where “R” refers to the number of repeat domains containing microtubule-binding domains)1. Understanding the various isoforms of tau proteins and their implications is essential for unraveling the mechanisms underlying pathologic conditions in neurodegenerative diseases.
Localization
Interestingly, tau isoform expression differs depending on the developmental stage and region of the brain. Only the shortest tau isoform (0N3R) is expressed during neurogenesis, hence it is known as ‘fetal tau’2.
In the non-pathological adult brain, the 3R and 4R isoforms are equally present. However, the variable N-terminal inserts exhibit differential expressions: 1N, 0N and 2N represent 54%, 37% and 9% of the total tau present respectively3. This distinct subcellular distribution of the N-terminal inserts suggests subunit-specific functions. For example, the 1N isoform is disproportionately enriched in the nucleus. Further, different proteins have different binding preferences for each isoform.
Isoform Characteristics
Research has illustrated that tau isoforms have variable properties. The 4R isoform assembles 2.5-3 times faster than the 3R isoform and has disparate levels of phosphorylation at various sites4. The 4R isoforms additionally have a higher binding affinity to microtubules and stimulate their assembly more efficiently5 – processes that are both regulated by phosphorylation6.
In contrast, the N-terminal domain has not been associated with microtubule binding affinity and tau self-association2. The N-terminal domain is involved with plasma membrane dynamics and microtubule spacing3. This section of the tau protein is less implicated in disease pathology; however, has been demonstrated to modulate tau aggregation propensity2.
Isoforms and Neurodegenerative Diseases
The C-terminal repeat domain of tau has been implicated in disease pathology. There are two primary streams of thought regarding its pathological role. One hypothesis supports that an abnormal abundance of either 3R or 4R is toxic, while others propose it is the imbalance in the ratio that is toxic4.
It has been observed that shifts in favor of one isoform or the other occur in neurodegenerative tauopathies. The brains of patients with Alzheimer’s disease (AD) and Frontotemporal dementia (FTD) contained a mix of 3R and 4R accumulations, while brains of patients with Corticobasal degeneration (CBD) and Pick’s Disease (PiD) both preferentially express specific tau isoforms (4R and 3R, respectively)4.
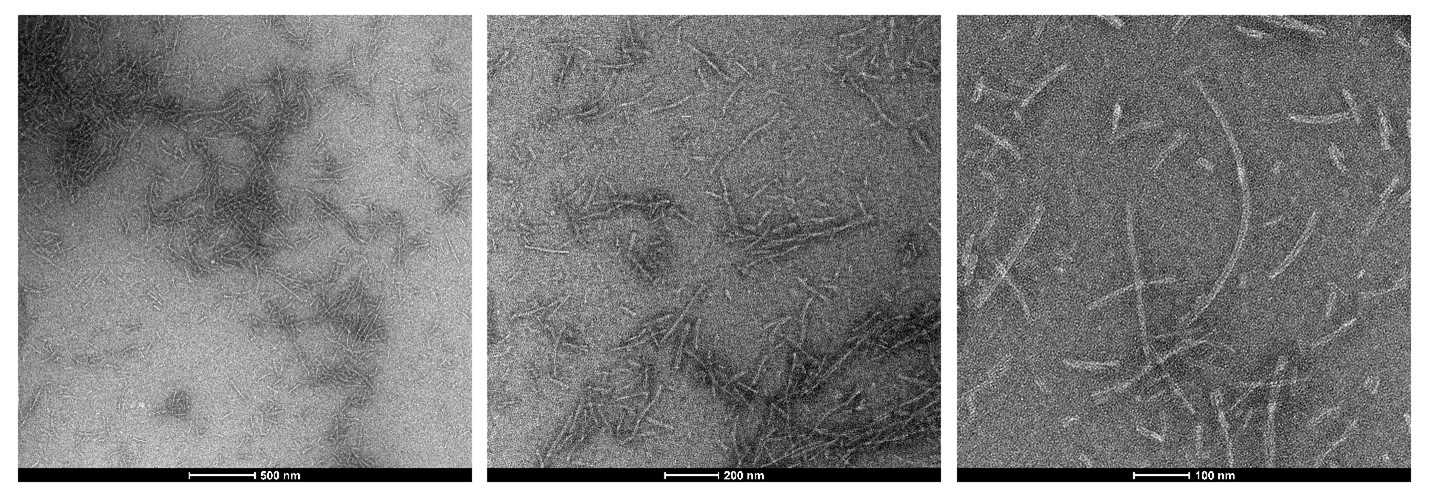
Figure 2: TEM of StressMarq’s Tau-352 (fetal 0N3R) (catalog#SPR-491) Tau Pre-formed Fibrils
Numerous studies have attempted to delineate how isoforms differ with respect to pathology and symptomatology. In one study, it was evident that each isoform possessed a unique profile7. An accumulation of the 4R hTau isoform resulted in impaired learning and memory, whereas an accumulation of 3R isoforms performed normally. Interestingly, the severity of the respective impairment was not proportional to overall tau levels, but to the number of microtubule-binding repeats7. Reported in another study, “expression of 3R isoform causes more axonal transport defects and locomotor impairments, whereas the 4R isoform correlated with greater neurodegeneration and impairments in learning and memory”8.
In an immunofluorescent analysis, researchers histologically characterized the differential accumulation of 3R and 4R isoforms in chronic traumatic encephalopathy (CTE) and Alzheimer’s disease (AD)9. Both diseases had a mix of 3R and 4R isoforms. Interestingly, a transition occurred where 4R predominated in mild disease state but 3R increased proportionally as pathological severity increased, correlating with extracellular tau pathology9.
In one hTau mouse model, selective 4R tau deposition was found in certain dementias along with exon 10-positioned MAPT mutations10. In this model, the mice with induced increased 4R-tau exhibited a greater abundance of phosphorylated and aggregated insoluble tau.
Yet, it is not apparent whether these variable pathological effects are a result of the 4R isoform or 3R isoform being more abundant, or due to an imbalance in the ratio. More research is needed to understand this relationship, as well as the significance, if at all, of the N-terminal domain.
Conclusion
Understanding the specific roles and interactions of different tau isoforms is crucial for developing targeted therapies aimed at preventing or slowing down the progression of tau-related neurodegenerative diseases. By unraveling the complexity of tau protein isoforms, scientists are paving the way towards a better understanding of the mechanisms underlying these devastating diseases and opening doors to potential treatments that may improve the lives of millions of people affected by them.
StressMarq is a pioneer in the development of tau proteins that facilitate the investigation of tau aggregation. StressMarq offers a range of fibrilized and oligomeric tau products, including various isoforms.
References
- Bachmann, S. et al. Differential effects of the six human tau isoforms: Somatic retention of 2N-tau and increased microtubule number induced by 4R-tau. Frontiers in Neuroscience. 2021; 15.
- Zhong, Q. et al. Tau isoform composition influences rate and extent of filament formation. Journal Biological Chemistry. 2012;287(24):20711-9.
- Strang, K. et al. MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Laboratory Investigation. 2019;99(7):912-928.
- Boyarko B, Hook V. Human Tau Isoforms and Proteolysis for Production of Toxic Tau Fragments in Neurodegeneration. Frontiers in Neuroscience. 2021;15:702788.
- Kosmidis, S. et al. Differential Effects of Tau on the Integrity and Function of Neurons Essential for Learning in Drosophila. Journal of Neuroscience. 2010;30(2):464-477.
- Feuillette, S. et al. Drosophila models of human tauopathies indicate that Tau protein toxicity in vivo is mediated by soluble cytosolic phosphorylated forms of the protein. Journal of Neurochemistry. 2010;113: 895-903.
- Vourkou, E. et al. Differential Effects of Human Tau Isoforms to Neuronal Dysfunction and Toxicity in the Drosophila CNS. International Journal of Molecular Sciences. 2022;23(21):12985.
- Sealey, M. et al. Distinct phenotypes of three-repeat and four-repeat human tau in a transgenic model of tauopathy. Neurobiology of Disease. 2017;105:74-83.
- Cherry, J.D., et al. Tau isoforms are differentially expressed across the hippocampus in chronic traumatic encephalopathy and Alzheimer’s disease. Acta Neuropathologica communications. 2021; 9,86.
- Schoch, K. et al. Increased 4R-Tau Induces Pathological Changes in a Human-Tau Mouse Model. 2016;90(5):941-7.


Leave a Reply