Alpha Synuclein and Mitochondrial Neurotoxicity
As people worldwide are living longer, the World Health Organisation (WHO) is now forecasting that neurodegenerative diseases will likely overtake cancer to become the second leading cause of death, after cardiovascular disease, by 2040. A hallmark of several neurodegenerative diseases is the aggregation of alpha synuclein into toxic fibrillar structures to form Lewy bodies (LB). These abnormal protein aggregations accumulate and propagate between neurons to cause LB dementia and Parkinson’s disease. Until now, there has been a lack of mechanistic information explaining how alpha synuclein aggregation may promote neuronal death.
A study by Horvath et al. uncovers the molecular mechanisms linking alpha synuclein aggregation to neurotoxicity. The researchers elucidated that alpha synuclein fibrils, along with Parkinson’s disease mutations, trigger increases in signature phosphoinositides of the plasma membrane (PM) — PI(4,5)P2 — through reinforced recruitment of PIP5K1γ via the critical regulator of vesicular traffic, ARF6. This is proposed to be a driving factor behind alpha synuclein aggregate pathology and increases mitochondrial Ca2+ and reactive oxygen species (ROS) levels, which in turn is thought to induce neuronal cytotoxicity. Modulating PM phosphoinositides and the effectors involved in the newly identified pathway represent potential targets to slow disease progression in LB-related neurodegenerative diseases.
Alpha synuclein fibrils deployed for Parkinson’s disease model
The researchers at the University of California and the University of Illinois employed two experimental models to uncover how alpha synuclein mediates neuronal cell death. One cellular model of synucleinopathy treated neuronal cells with StressMarq’s Human Recombinant Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322). Alpha synuclein fibrils promote the aggregation of endogenous alpha synuclein, propagate cell spread and induce neuronal dysfunction and degeneration — the defining features of Parkinson’s disease and LB dementia. Data was corroborated using a mouse model expressing the alpha synuclein mutant A53T, a genetic variant implied in familial Parkinson’s disease that possesses a higher propensity to aggregate.
Based on previously established findings, the study commenced by analyzing phospholipid species in each disease model using liquid chromatography-mass spectrometry. Isolated neurons treated with StressMarq’s Human Recombinant Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322) revealed a 50% increase in the levels of the phosphoinositide PI(4,5)P2 compared to control cells. This observation was confirmed with the Parkinson’s disease mouse model.
Spatial analysis using biosensor technology was performed to establish where these phosphoinositide increases occurred. Alpha synuclein overexpression or alpha synuclein disease mutations increased PM PI(4,5)P2 levels in different brain regions (hippocampus and substantia nigra) as well as non-neuronal cells. In contrast, increases in PI(4,5)P2 could not be induced with other pathological fibrils — namely, StressMarq’s Human Recombinant Tau-441 Wild-Type Pre-formed Fibrils (catalog# SPR-480) and Human Synthetic Amyloid Beta Pre-formed Fibrils (catalog# SPR-487).
PIP5K1γ is responsible for raised PM PI(4,5)P2 levels
To shed light on the relationship between alpha synuclein aggregation and increased PM PI(4,5)P2 levels, the researchers examined the kinase responsible for its synthesis from the P14P precursor — PIP5K1γ. Total Internal Reflection Fluorescence (TIRF) microscopy successfully tracked the dynamics of PIP5K1γ and revealed that translocation of PIP5K1γ to the plasma membrane was responsible for PI(4,5)P2 production. Normally residing in the cytosol, the migration of PIP5K1γ appeared to be stimulated by the presence of alpha synuclein.
Decoding alpha synuclein-dependent neurotoxicity
Subsequently, it was essential to understand how PIP5K1γ kinase was recruited to the PM. Looking further upstream of the molecular cascade, the researchers identified a small GTPase, ARF6, which was responsible for recruiting PIP5K1γ kinase to the PM. The ARF6 interaction with PIP5K1γ was shown to be indispensable in the production of increased levels of PI(4,5)P2 and was induced by both alpha synuclein fibrils and the disease mouse model.
Finally, the deleterious consequences of elevated levels of PI(4,5)P2 were investigated. Increased PI(4,5)P2 caused both direct and indirect cytotoxicity. Alpha synuclein aggregation promoted the accumulation of pathological phosphorylated alpha synuclein (pS129). Furthermore, the PI(4,5)P2 surges caused calcium release and increased ROS levels, leading to mitochondrial dysfunction and neurotoxicity.
Studying neurotoxicity using StressMarq products
Until now, the exact mechanistic information explaining how alpha synuclein aggregation leads to neuronal death has been poorly understood. By elucidating how alpha synuclein fibrils or related disease mutations may lead to aberrant PM phosphoinositide PI(4,5)P2 levels, Horvath et al., have identified new potential therapeutic targets for neurodegenerative diseases.
Chemical disruption of the effectors identified in the study demonstrated the potential to slow neurodegenerative disease progression. StressMarq’s Human Recombinant Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322) were indispensable in linking alpha synuclein to neurotoxicity via elevated lipid-rich PM complexes and increased mitochondrial Ca2+ and reactive oxygen species (ROS) levels. Other protein fibrils, including StressMarq’s Human Recombinant Tau-441 (2N4R) Wild-Type Pre-formed Fibrils (catalog# SPR-480) and Human Synthetic Amyloid Beta 1-42 Pre-formed Fibrils (catalog# SPR-487), could not reproduce the same effect on phosphoinositide signalling. These insights highlight therapeutic opportunities implicating PM phosphoinositide pathways for treating Parkinson’s disease and related neurodegenerative diseases.
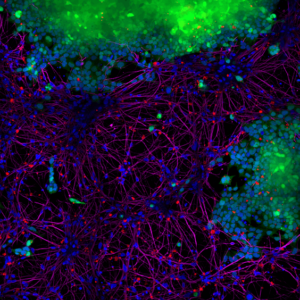
Figure 1. StressMarq’s Human Alpha Synuclein Pre-formed Fibrils (red) (catalog# SPR-322) are taken up by SH-SY5Y cells and transmitted to neuronal iPSCs within 14 days. Blue: Hoechst/DNA; Green: SHSY5Y-GFP; Red: Alpha Synuclein Pre-formed Fibrils -555 (catalog# SPR-322); Purple: Tubulin.
Related StressMarq products
Dedicated to accelerating neurodegenerative disease research, StressMarq excels in the manufacturing and characterization of high-quality reagents. Visit our website for details on our alpha synuclein, tau and amyloid beta constructs. Phosphorylation of alpha synuclein at Serine 129 is a standard marker for LB disease, which can be detected using StressMarq’s Alpha Synuclein Antibody (pSer129) (catalog# SMC-600).
References
- α-Synuclein-dependent increases in PIP5K1γ drive inositol signaling to promote neurotoxicity. Horvath, J.D. et al. Cell Reports. 2023.


Leave a Reply