The Role of Astrocytes in Parkinson’s Disease
Parkinson’s disease is a progressive neurodegenerative disorder marked by the loss of dopamine-producing neurons and the accumulation of alpha synuclein protein in the brain. Misfolded alpha synuclein aggregates are thought to trigger chronic inflammation via non-neuronal glial cells known as astrocytes. Astrocytes are essential for neuronal function, as they maintain central nervous system homeostasis by performing many vital support functions. These vital functions include regulating ion and water levels. Importantly, they also assist in maintaining the blood-brain barrier, clearing waste, and storing energy in the form of glycogen.
In reactive astrogliosis, a pathological stimulus results in astrocytes switching from a resting to an active state. Further, depending on the nature of the stimulus, the astrocytes’ reactive state can either be neurotoxic (A1), as observed post-mortem in Parkinson’s brain, or neuroprotective (A2), a state associated with ischemic injury. Understanding exactly how astrocytes switch into harmful, inflammatory states is crucial for unveiling new treatment strategies for Parkinson’s disease.
In a recent collaboration between UCL, The Francis Crick Institute, and the Korea Advanced Institute of Science & Technology (KAIST), D’Sa, et al. investigated the astrocytic response to alpha synuclein. Published in Science Advances, researchers generated cortical astrocytes and neurons from the induced pluripotent stem cells (hiPSC) of six healthy donors. Characterization of the astrocyte cultures confirmed correct cell type and functional maturity. This allowed the researchers to ensure that subsequent inflammatory challenges were assessed in a physiologically relevant human model. Cellular responses to alpha synuclein oligomers (alpha synuclein-O), aggregated in-house from monomers, were tested on three kinds of in vitro cell cultures: astrocyte-only, neuron-only, and astro-neuronal mixed cultures.
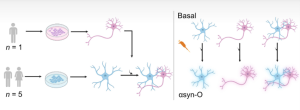
Figure 1. [Figure from D’Sa, et al.] hiPSC-derived astrocytes (blue) were generated from five healthy donors. Cortical neurons (pink) were derived from a sixth healthy donor. Cellular responses to stimulation with oligomeric alpha synuclein (alpha synuclein-O) in astrocyte-only, neuron-only, and astro-neuronal cultures were examined simultaneously.
Single-cell sequencing
Single-cell RNA sequencing was used on the three cultures to characterize genetic differences between cells, with or without alpha synuclein-O treatment. From this, eight distinct cell clusters were identified, two of which were astrocytic, and six neuronal. Subsequently, the two clusters were mapped specifically to astrocytic identities and were named astrocyte cluster (AC) 1 and 2. The AC1 cluster overexpressed cytokine and immune pathway genes with an inflammatory genetic profile. AC2 was enriched for morphogenesis and neurotrophic factors with a protective profile. Comparison with established iPSC-astrocyte profiles confirmed these subtypes. In particular, this comparison suggested that human astrocytes can assume preconfigured, divergent reactive states akin to classical A1 neurotoxic and A2 neuroprotective phenotypes in vivo.
Induction of reactive phenotypes using StressMarq’s neurodegenerative protein constructs
Next, D’Sa et al. observed internalization of exogenous alpha synuclein in cultured astrocytes, neurons, and co-cultures. Using a FRET biosensor for detection, researchers incubated these cultures with fluorescently labelled alpha synuclein monomers. Astrocyte cultures were found to induce higher levels of monomer uptake than neurons. However, similar levels of oligomer formation were detected within both cell types.
Notably, a previous study by the group found alpha synuclein-O treatment of primary astrocytes led to an inflammatory response. To induce this mechanism in the astrocyte hiPSC model, cells were treated with alpha synuclein-O. The researchers observed that treatment of the astrocytes with oligomers induced overproduction of reactive oxygen species (ROS), which was subsequently measured via dihydroethidium oxidation. Cellular death as a result of ROS overexpression was observed to be minimal, suggesting that an inflammatory response was induced. This experimental step also confirmed that the hiPSC-derived astrocytes could faithfully replicate the key biological stress responses observed in native cells.
StressMarq’s Alpha Synuclein Monomers (catalog# SPR-321) and Beta Synuclein Monomers (catalog# SPR-405) were tested in the current model, and were found to stimulate cytokine release. Compared to media-only cultures, incubation of hiPSC-derived astrocytes with alpha synuclein-O resulted in a significant release of proinflammatory cytokines (TNF-α, IL-6, IL-8, IL-1b and IFN-γ). Using bulk RNA sequencing on the treated cells, investigators conducted morphological analysis. This allowed them to conclude that the astrocytes displayed cytoskeletal remodeling and GFAP polarization upon oligomer exposure.
Together, these findings highlight the role of pathological alpha synuclein in converting astrocytes into inflammatory effectors. Subsequent bulk RNA sequencing revealed antiviral-like responses that paralleled the observed cytokine surge. Moreover, this suggests activation of innate immune pathways typically associated with viral recognition.
Astrocyte-neuron co-cultures spread neurotoxicity
In astro-neuronal co-cultures, exposure to alpha synuclein-O intensified inflammation and neuronal damage beyond astrocytes alone. In particular, co-cultures secreted higher cytokine concentrations and generated reactive oxygen species in both cell types, along with higher levels of cell death and impaired neuron firing. Analysis revealed a shift toward the inflammatory astrocyte subtype, AC1, underscoring how astrocyte state transitions directly undermine neuronal resilience.
ADAR1 up-regulation and global A-to-I editing
Following oligomer challenge of astrocyte-only and astro-neuronal co-cultures, cells displayed a markedly increased expression of ADAR1. This enzyme converts adenosine (A) to inosine (I) in double-stranded RNA. Isoform-specific splicing of ADAR shifted transcripts from the nuclear p110 to the interferon-inducible, cytoplasmic p150 variant under IFN-sensitive promoter control. Further, this switch may reflect a feedback mechanism that limits excessive antiviral signaling and maintains RNA homeostasis, while also amplifying global RNA editing activity within the cytoplasm during neuroinflammation.
Researchers found that astrocyte-containing samples exhibited increased editing site numbers and elevated editing rates in 3′ UTRs and coding regions, a sign of p150 activity in the cytoplasm. Therefore, genes that were edited and differentially expressed following alpha synuclein-O treatment were connected to the immune and viral response pathways. This association suggests that RNA editing may potentiate the inflammatory cascade. Analysis of postmortem brain samples from Parkinson’s patients revealed globally elevated A-to-I editing compared to controls. This study found that the most pronounced enrichment was in astrocyte-specific transcripts – linking this mechanism to human disease pathology.
Summary
The data presented in this study describes the successful establishment of a human iPSC model of astrocyte and neuron-like cells, in order to study the effects of alpha synuclein on the human brain. Using a variety of biochemical techniques, the researchers revealed that treatment with oligomeric alpha synuclein stimulated the overproduction of reactive oxygen species and the release of inflammatory cytokines. Notably, these neurotoxic astrocytes displayed antiviral-like responses to pathogenic alpha synuclein oligomers. Genetic analyses revealed that hiPSC-derived astrocytes upregulated ADAR1. This resulted in increased A-to-I RNA editing and a heightened propensity for chronic neuroinflammation.
Consistent with these findings, D’Sa, et al. also observed elevated A-to-I editing in postmortem brain samples from Parkinson’s disease patients. Collectively, this research provides key insights into the mechanisms underlying Parkinson’s disease-associated neurodegeneration. As well, these findings implicate ADAR1 upregulation and enhanced RNA editing as drivers of the neuroinflammatory cascade.
Related StressMarq products
As a world leading supplier of tools for neurodegenerative disease research, StressMarq is pleased to offer a wide range of optimized monomeric, oligomeric and fibrillar alpha synuclein, tau, and amyloid beta protein constructs. This diverse portfolio includes Gamma Synuclein Monomers (catalog# SPR-407) for Parkinson’s and Alzheimer’s disease studies. Visit our Product Citations page to learn more about how StressMarq products are being used to drive advances in neurodegeneration research.
References
- Astrocytic RNA editing regulates the host immune response to alpha-synuclein. D’Sa, K. et al. Sci. Adv. 2025.


Leave a Reply