Gut Alpha Synuclein Aggregation in Parkinson’s Disease
Parkinson’s disease (PD) is characterized by the accumulation of intracellular inclusions of alpha synuclein in the brain, a process that results in neurodegeneration. Here, alpha synuclein fibrils are taken up by neurons, inducing aggregation of endogenous alpha synuclein and subsequent aggregate spreading throughout the brain.
Motor deterioration is among the predominant symptoms of advanced PD. However, decades before these motor symptoms appear, some PD patients suffer from gastrointestinal problems such as constipation. Indeed, in addition to the 100 million neurons in the brain, the gut contains 500 million neurons, connected to the brain via nerves in the nervous system. Evidence from animal models implicates the processing of alpha synuclein fibrils in the trafficking of fibrils from the enteric nervous system (ENS) to the central nervous system (CNS).
Recognizing the lack of in vitro experimental data conducted in primary enteric neurons, a new study published in Scientific Reports by Bindas et al., has examined how enteric neurons uptake and retain alpha synuclein fibrils in an environment containing gut luminal contents. These results provide insight into the cellular mechanisms underlying alpha synuclein aggregation in enteric neurons and contribute to the growing awareness of the importance of ENS degeneration in PD.
Monitoring alpha synuclein aggregation in enteric neurons
Pathological alpha synuclein inclusions in PD are characterized by elevated alpha synuclein aggregation. Due to limited data, it has remained unclear whether alpha synuclein PFFs (pre-formed fibrils) could induce aggregation of alpha synuclein in enteric neurons. In the paper by Bindas et al., StressMarq’s fluorescently-tagged Human Recombinant Alpha Synuclein Pre-formed Fibrils: ATTO 594 (catalog# SPR-322-A594), was utilized in tandem with live- and fixed-cell imaging, allowing PFF uptake and processing to be tracked over time within in vitro experimental models employing both cortical and enteric neurons.
It was found that both the cortical and enteric neurons exhibited alpha synuclein aggregation. However, the neurons responded differently depending on the dose administered. In cortical neurons, alpha synuclein aggregation increased in accordance with increasing PFF dosage; only the highest dose administered of alpha synuclein PFFs resulted in alpha synuclein aggregation. Interestingly, uptake in enteric neurons could be impacted by using inhibitors of protein degradation pathways (targeting endosomes and lysosomes). The trafficking of the PFFs from the cell surface to lysosomes, and then to inclusions could explain how alpha synuclein is transferred to neighbouring cells.
In addition, the impact of alpha synuclein aggregation on neuron function was studied. Specifically, the highly motile structures responsible for interacting with the extracellular environment — filopodia of the growth cone — were found to be affected in enteric neurons with PFF addition, leading the researchers to propose that PFF dosage may result in morphological changes to growth cones. Other neuronal processes, such as neurotransmitter release and information processing, remained unaffected.
The influence of gut contents
The luminal contents of the gut have also been shown to play a role in PD pathology. The researchers investigated the presence and effects of lipopolysaccharide (LPS) and butyrate in the gut environment. It was found that LPS could induce alpha synuclein aggregation, while butyrate was shown to have a protective effect against LPS. Co-administration could alleviate the detrimental effects of LPS. Ultimately, further work is needed to understand the intracellular mechanisms underlying the modulatory effects of gut contents on enteric neurons.
StressMarq’s fluorescently-tagged alpha synuclein fibrils
Growing evidence suggests that gut problems may be an early warning sign of an increased risk of developing Parkinson’s disease. StressMarq’s fluorescently-tagged Human Recombinant Alpha Synuclein Pre-formed Fibrils: ATTO 594 (catalog# SPR-322-A594) were instrumental in this study to determine how neurons in the gut and brain uptake alpha synuclein differently in a dose-dependent manner and observing the modulation of fibril uptake by gut environmental factors. In conclusion, characterizing alpha synuclein fibril uptake and retention in enteric neurons is a step towards understanding how early gut symptoms of Parkinson’s disease develops into pathological disease progression throughout the brain.
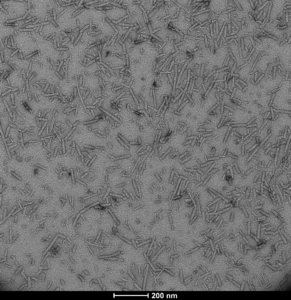
Figure 1. Transmission electron microscopy image of Human Recombinant Alpha Synuclein Pre-formed Fibrils: ATTO 594 (catalog# SPR-322-A524)
Related StressMarq products
StressMarq is the leading commercial supplier of Alpha Synuclein Pre-formed Fibrils (PFFs), Oligomers, & Monomers. These highly characterized alpha synuclein products aid researchers in deciphering the molecular pathways that give rise to the systemic effects of neurodegenerative diseases. Antibodies can also be used to monitor endogenous and disease-causing fibrillar forms of alpha synuclein. StressMarq’s newly released Alpha Synuclein (Aggregate-Specific) [2F11] Antibody (catalog# SMC-617) specifically detects fibrillar and oligomeric forms of alpha synuclein and has been demonstrated to detect alpha synuclein aggregation in gut tissue samples.
References
- Aggregation of alpha-synuclein in enteric neurons does not impact function in vitro. Bindas A. J. et al., Scientific Reports. 2022; 12 (22211).

Leave a Reply