Intercellular Transmission of Alpha Synuclein in Parkinson’s Disease and Other Synucleinopathies
Alpha synuclein is a major component of Lewy bodies and Lewy neurites, the intraneuronal protein inclusions characteristic of Parkinson’s disease (PD) and other synucleinopathies. While Lewy pathology initially appears in the olfactory bulbs and dorsal motor nucleus of the vagus nerve (a cranial nerve with both sensory and motor functions), it progressively affects other regions of the brain and nervous system. This important observation, first made by Braak et al. almost 20 years ago, spawned the hypothesis that alpha synuclein propagation has a central role in PD pathogenesis1. As a result, numerous studies have focused on resolving the mechanisms underlying the spread of alpha synuclein, many of which are also implicated in the propagation of other aggregation-prone proteins such as amyloid-β, tau, huntingtin, and superoxide dismutase 12.
Cellular release of alpha synuclein involves multiple mechanisms
Due to the lack of a secretory signal peptide, alpha synuclein was originally thought to be purely an intracellular protein. However, its detection in plasma, saliva, urine, and other biological fluids from both healthy controls and patients with PD soon proved this to not be the case. Further supporting evidence for alpha synuclein secretion came from the finding that neuronal cells release alpha synuclein monomers and aggregates into culture medium, a process which was subsequently shown to occur via endoplasmic reticulum/Golgi-independent (unconventional) exocytosis3.
Many more molecular mechanisms have now been linked to alpha synuclein secretion. These include other forms of unconventional exocytosis, which are reported to exploit the misfolding-associated protein secretion pathway (a quality control measure for transporting misfolded cytosolic proteins into the extracellular space) and to utilize exophagy (a secretory process for removing autophagic vesicles). In addition, alpha synuclein is known to be secreted via exosomes and can be transferred via tunneling nanotubes, the long F-actin-based membranous channels that connect different cells with one another4.
Alpha synuclein uptake is similarly complex
Following its release from neurons, alpha synuclein may be degraded by extracellular proteases (e.g., matrix metalloproteases, plasmin, and neurosin) or internalized by neighboring cells. Currently, the mechanisms by which alpha synuclein monomers are internalized remain unclear, but endocytosis, direct translocation, and lipid raft-mediated methods have all been suggested. In contrast, cellular uptake of oligomeric and fibrillar alpha synuclein has been shown to be endocytic and is thought to require specialized receptors. These include TLR2 and Cx32, which interact with alpha synuclein oligomers, and LAG3 and FcγRIIIb, which are implicated in the uptake of alpha synuclein fibrils4.
Experimental proof for intercellular transmission of alpha synuclein
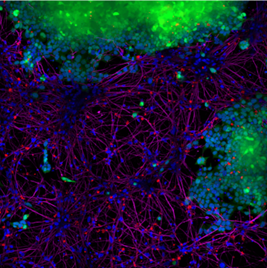
StressMarq’s Alpha Synuclein PFFs (catalog# SPR-322) were shown to be taken up by SH-SY5Y cells and transmitted to neuronal iPSCs within 14 days. Blue: Hoechst/DNA; Green: SHSY5Y-GFP; Red: Alpha Synuclein PFFs; Purple: Tubulin.
A growing body of evidence suggests alpha synuclein pathology to be transmissible intercellularly. For example, co-culturing two populations of human dopaminergic neuronal cells, one expressing myc-tagged alpha synuclein, has been shown to cause the formation of alpha-synuclein inclusions in the acceptor cells within just 24 hours5. And host-to-graft transmission of alpha synuclein has been observed in vivo6.
Additionally, grafting embryonic dopaminergic neurons into PD patients has led to the development of alpha synuclein inclusions in the donor cells, indicating that pathology is conferred from the diseased tissue7.
As researchers continue to learn more about the mechanisms involved in alpha synuclein transmission, an effective cure for Parkinson’s disease and other synucleinopathies comes ever closer.
Supporting research
StressMarq offers a broad range of reagents for studying alpha synuclein pathology. These include Human Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322), which have been shown to successfully transmit from cell to cell. These can be used with Human Alpha Synuclein Monomers (catalog# SPR-321) for seeding fibril formation in vitro.
References
- Staging of brain pathology related to sporadic Parkinson’s disease, Braak H et al. Neurobiol Aging. 2003 Mar-Apr;24(2):197-211
- The propagation of prion-like protein inclusions in neurodegenerative diseases, Goedert M et al, Trends Neurosci. 2010 Jul;33(7):317-25
- Intravesicular localization and exocytosis of alpha-synuclein and its aggregates, Lee HJ et al, J Neurosci. 2005 Jun 22;25(25):6016-24
- Molecular events underlying the cell-to-cell transmission of α-synuclein, Choi YR et al, FEBS J. 2021 Dec;288(23):6593-6602
- Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein, Desplats P et al, Proc. Natl. Acad. Sci. 2009;106(31): 13010–13015
- α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells, Hansen C et al. J Clin Invest. 2011 Feb;121(2):715-25
- Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease, Kordower JH et al, Nat Med. 2008 May;14(5):504-6


Leave a Reply