Nuclear Tau and Lamins in Alzheimer’s Disease Progression
Alzheimer’s disease (AD) is the most prevalent, progressive age-related neurodegenerative disorder. Scientists around the world are working to find the causes of this complex disease and develop effective treatments. The hippocampus is one of the primary brain regions affected by AD and its pathological hallmarks, amyloid beta plaques and tau tangles, are often found in this region.
Nuclear Origin of Alzheimer’s
Recent studies demonstrate the nuclear origin of Alzheimer’s. Tau plays a major role in disease pathology and is considered a multifunctional protein. Beside its role as a microtubule -associated protein, nuclear localization of tau has been observed in neuronal and non-neuronal cells1. Tau interacts with the neuronal genome to prevent DNA damage under physiological or heat-shock conditions and promotes chromosome stability2. Specifically, tau regulates the organization of neuronal pericentromeric heterochromatin which plays a critical role in regulating chromosome segregation, genomic stability and gene expression3. In AD onset and progression, tau gradually disappears from neuronal nuclei and accumulates in the cytoplasm4.
What is Nuclear Lamin?
The nuclear lamin (NL) is a dense fibrillar network inside the nucleus of cells that connects the inner membrane to chromatin, providing a link between the cytoplasm and the genome. NL also provides mechanical support and is involved in most cellular events including DNA replication, RNA transcription, nuclear and chromatin organization, cell cycle regulation, cell development and differentiation, nuclear migration, and apoptosis. It is composed of lamins and nuclear lamin-associated membrane proteins5.
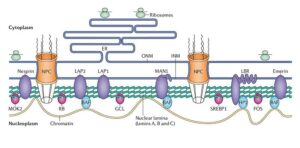
Structure and function of the nuclear lamina. Coutinho et al. Immunity & Ageing 2009. Henrique Douglas M Coutinho , Vivyanne S Falcão-Silva, Gregório Fernandes Gonçalves and Raphael Batista da Nóbrega / CC BY (https://creativecommons.org/licenses/by/2.0)
What are the Lamins?
The lamins are type V intermediate filaments divided into 2 subtypes, type A (lamin A, C) and type B (lamin B1, B2)6. In vivo, B-type lamins are expressed in all cells, while A-type lamins are expressed only in differentiated cells7. Mutations in lamins A and C have been shown to cause a variety of diseases known as laminopathies8. Studies have demonstrated that B- type lamins are involved in Alzheimer’s disease onset which suggests that AD could be considered an acquired neurodegenerative laminopathy9,10. In a transgenic human tau Drosophila model, researchers observed that tau-induced lamin dysfunction leads to heterochromatin relaxation and neuronal cell death11.
Nuclear Lamins and Heterochromatin
Heterochromatin is a densely packed form of DNA, mainly located at the periphery of the nucleus. It is transcriptionally inactive and plays a significant role in gene regulation and the protection of chromosome integrity. Heterochromatin is subdivided into densely packed constitutive heterochromatin which is found at centromeric and pericentromeric chromosomal regions, and less condensed facultative heterochromatin located in the chromosomal arms12. The nuclear lamins interact with chromatin at the nuclear periphery forming the lamin-associated chromatin domains (LADs) which are involved in genome organization and gene expression. LADs mainly contain genes that are silenced or weakly expressed and they are enriched in repressive chromatin marks such as H3K9me2/3 and H4K20me313. Increased expression of the epigenetic marker H4K20me3 is associated with loss of peripheral or total heterochromatin in laminopathies.
Neuronal Cell Cycle Re-entry in AD
Several studies suggest that post-mitotic neurons of an adult nervous system are fully differentiated and incapable of undergoing cell division. After differentiation they enter the quiescent G0 phase of the cell cycle. Nevertheless, in neurodegenerative diseases, aged post-mitotic neurons can re-enter the cell cycle. The AD brain suffers significant synaptic and neuronal loss and is affected by the accumulation of the toxic tau aggregates. In response to DNA damage, post-mitotic neurons exit the G0 phase and re-enter the cell cycle which leads to apoptosis14.
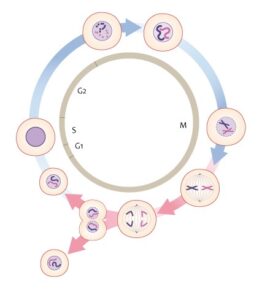
The Cell Cycle. Genomics Education Program / CC BY (https://creativecommons.org/licenses/by/2.0)
Nuclear Tau and Lamins Involved in AD Progression
A recent study published in the International Journal of Molecular Sciences, demonstrated that nuclear tau and lamins in hippocampal neurons are involved in the reorganization of the nucleo-cytoskeleton and the formation of neurofibrillary tangles. The researchers analyzed alterations of the 4 lamin subtypes in CA1 and CA3 hippocampal regions of AD brain tissues and their relationship with phosphorylated tau and centromeric and pericentromeric heterochromatin.
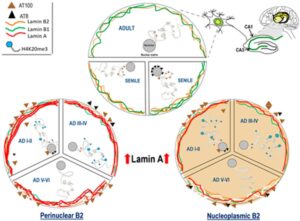
Nuclear dynamics of Tau, nuclear lamins, and H4K20me3 in the hippocampus of adult, senile AD I-II, AD III-IV, and AD V-VI Braak stages. Source: https://www.mdpi.com/1422-0067/21/5/1841/html
The study showed that:
- During AD progression, phosphorylated tau gradually disappears from the nucleus and moves to the cytoplasm. The decrease of nuclear tau affects the pericentromeric heterochromatin stability.
- Lamin A is highly expressed in hippocampal neurons from early to late AD stages.
- Lamin B2 which is mostly perinuclear, redistributes in the nucleoplasm in AD. These changes determine 2 types of neurons: those with reinforced perinuclear Lamin B2 and those with nucleoplasmic lamin B2.
- Nuclear lamin and nuclear tau changes are associated with constitutive heterochromatin modifications.
The results demonstrate the involvement of nuclear lamins in Alzheimer’s disease progression. The re-entry of old post-mitotic neurons into the cell cycle leads to their death because of their complex nucleo-cytoskeleton. The nucleo-cytoskeletal stress triggers the expression of lamin A to protect the genome and reorganize the nucleo-cytoskeleton. Nuclear tau and lamins participate in the nuclear pathological transformation of neurons at early AD which affects neural plasticity and leads to synaptic dysfunction.
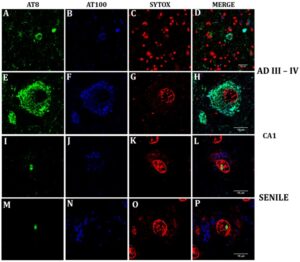
Confocal analysis of nuclear and cytoplasmic phosphorylated Tau in senile CA1 hippocampal neurons and at AD III-IV Braak stages.
StressMarq’s rabbit tau monoclonal antibody (SMC-601) was used in this study as it recognizes the same epitope (pSer202/pThr205) as the AT8 phospho tau antibody. The researchers used this antibody (Clone AH36) to analyze nuclear and cytoplasmic phosphorylated tau in senile and AD hippocampal neurons and showed for the first time the nuclear presence of pTau (Ser202/Thr205) in aging hippocampal neurons.
REFERENCES
- Nuclear Tau and Its Potential Role in Alzheimer’s Disease. Bukar Maina, et al. (2016) Biomolecules 6: 9.
- Nuclear tau, a key player in neuronal DNA protection. Sultan A. et al. (2011) J Biol Chem. 286(6):4566‐4575.
- Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin. Mansuroglu, Z. et al. (2016) Sci. Rep. 6: 33047.
- Altered Machinery of Protein Synthesis in Alzheimer’s: From the Nucleolus to the Ribosome. Hernández-Ortega et al. (2016) Brain Pathol. 26(5): 593‐605.
- The nuclear lamina and its functions in the nucleus. Gruenbaum et al. (2003) International Review of Cytology, 226: 1-62.
- The lamin protein family. Dittmer, T.A., Misteli, T. (2011) Genome Biol 12:222.
- Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. Moir R.D. et al. (2000) J Cell Biol. 151(6):1155-1168.
- How do mutations in lamins A and C cause disease? Worman H.J. et al. (2004) J Clin Invest. 113(3):349-351.
- Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Frost B. et al. (2016) Curr. Biol. 26: 129–136.
- Alzheimer’s disease: An acquired neurodegenerative laminopathy. Frost B. (2016) Nucleus 7(3): 275–283.
- Hippocampal LMNA Gene Expression is Increased in Late-Stage Alzheimer’s Disease. Méndez-López I. et al. (2019) Int J Mol Sci. 20(4):878.
- The Nuclear Lamina as an Organizer of Chromosome Architecture. Shevelyov Y.Y. et al. (2019) Cells. 8(2):136.
- Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. van Steensel B. et al. (2017) Cell 169(5):780-791.
- Cell division in the CNS: protective response or lethal event in post-mitotic neurons? Yang Y, Herrup K. (2007) Biochim Biophys Acta. 1772(4):457‐466.


Leave a Reply