Protein Aggregates as Early Biomarkers for Neurodegeneration
The formation of misfolded protein aggregates is a hallmark of neurodegenerative diseases. Alpha synuclein oligomers and fibrils incorporate into abnormal neurotoxic intracellular inclusions in Parkinson’s disease (PD). Similarly, intracellular neurofibrillary tangles of tau protein and extracellular amyloid beta proteins contribute to the progression of Alzheimer’s disease (AD). These oligomeric protein aggregates are early biomarkers of the neurodegenerative process, and are typically present long before symptoms develop.
Detecting oligomeric protein forms has proved notoriously difficult as histological stains fail to stain oligomeric structures, while other methods lack sensitivity and specificity when detecting oligomers from biospecimens. A novel assay developed by world-leading researchers at the University of Edinburgh (in collaboration with StressMarq Biosciences), has quantified alpha synuclein oligomers for the first time, achieving greater sensitivity and specificity for oligomers from patient cerebrospinal fluid (CSF) and other biological samples. This technique could potentially be used to evaluate pharmacological agents that target aggregation and clinical therapeutic interventions.
STAPull assay detects protein-specific aggregates
The assay developed in the paper by Saleeb et al., — named single-molecule two-colour aggregate pull-down (STAPull) — quantifies aggregated protein species using antibody detection instead of thioflavin T (ThT) stain, which remains unreactive to oligomeric structures as they lack extended β-sheet structures. The researchers employed a biotinylated capture antibody, supported by biotin and streptavidin bound to a PEGylated glass surface.
StressMarq’s extensively characterized alpha synuclein monomers (catalog# SPR-321), kinetically-stable oligomers (catalog# SPR-484) and pre-formed fibrils (PFFs) (catalog# SPR-322) were captured using a biotinylated anti-alpha synuclein antibody. Oligomers were detected when two orthogonally labelled detection antibodies targeted the same epitope, resulting in two-colour colocalization. Monomers of alpha synuclein produced a single-color signal. Single-molecule level detection was performed using total internal reflection fluorescence (TIFR) microscopy.
Unambiguous oligomeric detection of alpha synuclein in biological samples
With a significantly reduced background signal, the STAPull assay possesses a better limit of detection than that of its predecessors: 600-fold better than routinely used ThT fluorescence assays (using plate-reader formats) and 3-fold higher than a similar pull-down assay (single-molecule pull-down: SiMPull). Additionally, the STAPull assay displays no cross-reactivity between capture antibodies and protein targets. However, the platform could be adapted to detect tau aggregates specifically by using an anti-tau capture antibody. Additionally, unlike SAVE (single aggregate visualization by enhancement) imaging, which employs ThT detection and TIRF microscopy, the STAPull can differentiate between protein-specific oligomers and fibrils.
Non-specific interactions and background signals can make identifying oligomeric protein species in a complex biological sample a challenging task. To test oligomeric detection in biological samples, the authors employed induced pluripotent neurons stem cells derived from a patient with PD harbouring a triplication in the alpha synuclein gene, a condition known to produce elevated levels of alpha synuclein aggregates. Using the STAPull assay, a significant increase in alpha synuclein aggregates in these samples was successfully characterized. Moreover, CSF samples from PD patients were also determined to contain elevated levels of alpha synuclein aggregates.
StressMarq products for developing neurodegenerative diagnostic assays
Detecting sub-populations of aggregated proteins is the first step in identifying early biomarkers and diagnosing neurodegenerative diseases. The STAPull assay overcomes historical constraints of specificity and sensitivity for quantifying protein aggregates in biological samples and biospecimens. StressMarq’s alpha synuclein monomers (catalog# SPR-321), alpha synuclein oligomers (catalog# SPR-484) and alpha synuclein pre-formed fibrils (PFFs) (catalog# SPR-322) were crucial for confirming the specific detection of each protein sub-population. Furthermore, therapeutic and diagnostic applications in neurodegenerative diseases and other protein aggregation diseases may benefit greatly from the incorporation of the STAPull assay protocol.
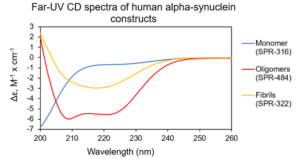
Figure 1. Far-UV CD spectra of human alpha synuclein monomers (catalog# SPR-321), kinetically-stable alpha synuclein oligomers (catalog# SPR-484) & alpha synuclein pre-formed fibrils (PFFs) (catalog# SPR-322).
Related StressMarq products
Dedicated to providing cutting-edge products for neurodegenerative disease research, StressMarq is a leader in protein oligomerization. StressMarq’s tau monomers and tau pre-formed fibrils (PFFs) are critical in studying tau aggregation in Alzheimer’s disease. In addition, amyloid beta peptide (Aβ) can also aggregate and form oligomers, fibrils and ultimately Aβ plaques in Alzheimer’s disease. StressMarq offers amyloid beta monomers, amyloid beta oligomers and amyloid beta PFFs for various in vitro applications.
References
- Two-color coincidence single-molecule pull-down for the specific detection of disease-associated protein aggregates. Saleeb, R, S., Leighton, C., Lee, J, O’Shaughnessy, J., Jeacock, K., Chappard, A., Cumberland, R., Ball, S. R., Sunde, M., Clarke, D. J., Piché, K. *, McPhail. J. A*, Louwrier, A.*, Angers, R., Gandhi, S., Downey, P., Kunath, T. and Horrocks, M. H. Two-color coincidence single-molecule pull-down for the specific detection of disease-associated protein aggregates | bioRxiv. Note: This is a preprint. The article has not been peer-reviewed by a journal.
*Three members of the StressMarq team collaborated in this research: Ariel Louwrier: President and CEO of StressMarq Biosciences; Jacob McPhail: Lead R&D Scientist at StressMarq Biosciences; Kirstin Piché: R&D Laboratory Technician at StressMarq Biosciences.

Leave a Reply