Targeting Alpha Synuclein Aggregation in Early Parkinson’s Disease
A Potential Therapeutic Strategy
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that affects millions of people worldwide. A combination of genetic, environmental, and aging factors may lead to disease progression, but the real cause is still unknown. Currently, there is no cure and only a few effective treatments that can improve motor symptoms. Scientists have been investigating the potential causes of this neurodegenerative disease and developing new therapies.
Alpha synuclein has a central role in the disease pathogenesis as its abnormal accumulation and aggregation leads to Lewy bodies and Lewy neurites formation, the main hallmark of PD. Alpha synuclein oligomeric and fibrillary forms are found to be neurotoxic and act in a prion-like manner as they can spread in different brain areas. Several studies have shown that targeting those aggregates has become a critical step in the development of new therapeutic strategies. Recombinant alpha synuclein monomers are incubated and form alpha synuclein pre-formed fibrils (PFFs) which can be used to model PD. Adding these PFFs to cells or animals can induce aggregation of the endogenous alpha synuclein as well as Lewy body pathology.
Alpha synuclein aggregates interact with neuronal cell membranes
Alpha synuclein-membrane interactions have been the subject of several studies which indicated that toxic oligomeric and fibrillary forms of the protein can cause a rapid destruction of the membranes. Specifically, different aggregates can alter membrane morphology and permeability. These interactions seem to affect both protein and membrane properties as they lead to membrane disruption and affect the kinetics of alpha synuclein fibril formation2.
Researchers from California State University in LA used alpha synuclein oligomers and PFFs to study how these aggregates affect neuronal cell membranes and suggested potential strategies to prevent neuronal damage in PD patients1,3.
In their first study, they exposed live SH-SY5Y neuroblastoma cells to alpha synuclein oligomers and used Scanning Ion Conductance Microscopy (SICM) to observe morphological membrane changes. Higher concentrations of alpha synuclein oligomers destabilized the lipid bilayer by opening transient pores on the membrane which eventually led to membrane disruption3.
In a second study, recently published in ACS Chemical Neuroscience journal, the researchers used StressMarq’s human alpha synuclein PFFs (catalog# SPR-317) to investigate the effects of fibril exposure on SH-SY5Y cell membranes1.
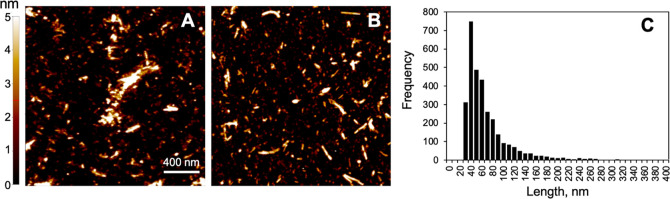
Figure 1. AFM characterization of PFFs. (A, B) Representative AFM images (2 μm × 2 μm) of α- Syn PFFs adhered to a mica substrate. Images were first- order flattened using XEI. (C) Histogram of the lengths of PFFs imaged with AFM (n = 3290). Source: Feng, C. et al. ACS Chem. Neurosci. 2022; 13(24):3547-3553.
The effects of alpha synuclein PFFs on neuroblastoma cell membranes
Christina Feng and her team investigated the effects of alpha synuclein fibril exposure on SH-SY5Y neuroblastoma cell membranes using Scanning Ion Conductance Microscopy (SICM). Specifically, different concentrations of alpha synuclein PFFs were added to neuroblastoma cells for 48 hours. Then, the cell membranes were observed with SICM, a technique with the ability to produce high resolution non-contact imaging of the surface of living tissues and cells in real time. The SICM images indicated a significant increase in membrane roughness as fibril concentration increased.
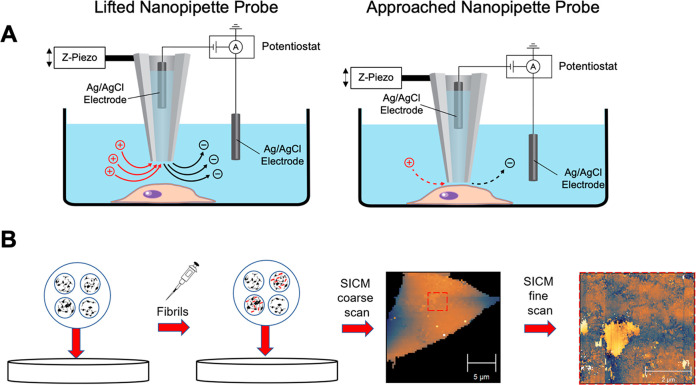
Figure 2. (A) Schematic of SICM. (B) Flowchart illustrating the SICM experiment procedure. PFFs (red) are added to wells containing SH-SY5Y cells and incubated for 48 h. SICM was then used to image cells, starting with a coarse whole-cell scan (64 × 64 pixels) and followed by multiple fine scans (128 × 128 pixels). Source: Feng, C. et al. ACS Chem. Neurosci. 2022; 13(24):3547-3553.
The researchers also assessed cell viability and cytotoxicity to further explain the SICM images. The XTT viability assay showed a small but statistically significant decrease in cell viability and the LDH cytotoxicity assay only showed a small increase in LDH release, but they were both independent of the fibril concentration. The assay results suggest that PFFs may affect membranes and disrupt cell function without leading to cell death. The findings of this study indicate that targeting pathological alpha synuclein fibrils before their accumulation and further aggregation might prevent neuron loss in early PD stages and could lead to the development of a promising therapeutic strategy1.
StressMarq’s fibrillary proteins support Parkinson’s disease research
StressMarq offers a wide variety of Alpha Synuclein Monomers, Oligomers and Pre-formed fibrils, useful for modelling Parkinson’s disease because of their ability to induce aggregation and pathology in different cellular and animal models. These include Type 2 Human Alpha Synuclein Pre-formed Fibrils (catalog# SPR-317) which have been shown to interact with neuronal membranes without causing cell death.
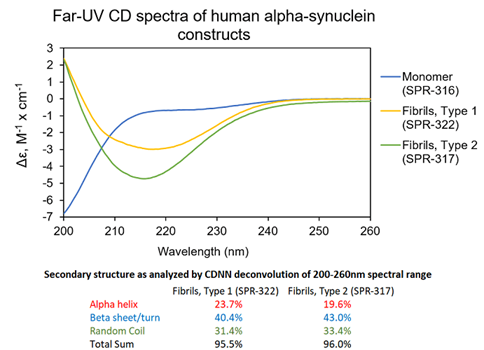
Figure 3. UV-CD data suggests StressMarq’s Alpha Synuclein Pre-formed Fibrils (PFF), Type 1 (cat#SPR-322) and Type 2 (cat#SPR-317) both have a high beta sheet/turn content, yet do have small secondary structure differences. StressMarq’s Alpha Synuclein Monomers (cat#SPR-316) show a strong negative signal at 200 nm indicative of a disordered protein state (low secondary structure content). For this experiment, pre-formed fibrils (PFF) were subjected to 10 cycles of sonication prior to UV-CD to ensure solubility prior to measurement.
In addition to alpha synuclein fibrillar proteins, StressMarq offers a range of antibodies, proteins, kits, and small molecules for Parkinson’s disease research, including Amyloid Beta and Tau.
References
- Observation of α‑Synuclein Preformed Fibrils Interacting with SHSY5Y Neuroblastoma Cell Membranes Using Scanning Ion Conductance Microscopy, Feng, C. et al., ACS Chem. Neurosci. 2022; 13(24):3547-3553.
- The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation, Zhu, M. et al., J. Biol. Chem. 2003; 278: 40186– 40197.
- Real-Time Characterization of Cell Membrane Disruption by α-Synuclein Oligomers in Live SH-SY5Y Neuroblastoma Cells, Parres-Gold, J. et al. ACS Chem. Neurosci. 2020; 11(17): 2528−2534.


Leave a Reply