Tau in Alzheimer’s Disease Diagnostics
Tubulin-associated unit (tau) is a microtubule-associated protein (MAP) found mainly in neurons of the central nervous system. Here, it functions to regulate microtubule assembly and stability, as well as modulating axonal maintenance and transport and controlling neurite outgrowth1. Alterations in tau have been linked to more than 20 different neurodegenerative conditions, collectively referred to as tauopathies2. These include Parkinson’s Disease, progressive supranuclear palsy (PSP), Pick’s disease (PiD), and Alzheimer’s disease. Because these conditions share many similarities in their clinical presentation, diagnosing them is challenging. To address this problem, tau is being investigated as a biomarker with significant potential to improve the reliability of disease diagnosis.
How could tau be used to diagnose Alzheimer’s disease?
Alzheimer’s Disease is the most common tauopathy, characterized by the presence of amyloid β (Aβ) deposits and neurofibrillary tangles in the brain. These play a central role in Alzheimer’s disease pathogenesis, with the latter forming the basis of a staging system that is widely used post-mortem to confirm the extent of disease progression3. Because neurofibrillary tangles are composed mainly of hyperphosphorylated tau, determining the nature and level of tau phosphorylation shows considerable promise as a means of diagnosing Alzheimer’s disease.
However, there are many further ways that tau might be exploited to diagnose Alzheimer’s disease and other tauopathies. For instance, research efforts have linked mutations in the MAPT gene to frontotemporal dementia; shown MAPT polymorphisms to be associated with an increased risk of developing syndromes with parkinsonism; and demonstrated altered alternative splicing of MAPT mRNA to correlate with an irregular pattern of tau isoforms in the brain4.
What are different approaches to measuring tau?
Although tau is widely monitored by post-mortem immunohistochemical (IHC) analysis of brain tissue, its detection in body fluids or in tissues that are more readily accessible for biopsy would support early diagnosis and allow for intervention aimed at slowing disease progression. Cerebrospinal fluid (CSF), blood, and oral, ocular, and olfactory secretions have all been evaluated for their suitability as diagnostic tools for Alzheimer’s disease, predominantly using commercial ELISAs, whilst biopsy material derived from olfactory epithelium has been assessed by IHC5.
Complementing these studies, antibody arrays, bead-based assays, IP-MS with MALDI-TOF mass spectrometry, and NMR spectroscopy have all been employed to study multiple protein targets of relevance to Alzheimer’s disease research. Most recently, gene expression arrays have been used to compare transcriptional differences between skin punch biopsies taken from healthy human subjects and individuals with Alzheimer’s disease, providing insights that may ultimately lead to the identification of additional Alzheimer’s disease biomarkers6.
Supporting the study of tau and its role in Alzheimer’s disease
We’ve developed a comprehensive portfolio of reagents to support researchers studying tau and its role in Alzheimer’s disease. This includes a wide selection of high-quality tau proteins and antibodies, as well as our active tau preformed fibrils (PFFs) and filaments. When active tau PFFs are combined with active tau monomers, thioflavin T assays demonstrate an increase in fluorescence that is indicative of tau fibrillization. Our active tau PFFs have also been successfully used to seed the process of tau aggregation in a mouse model system.
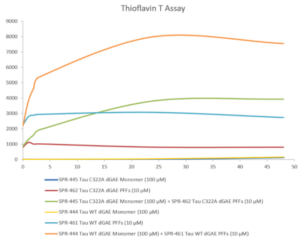
Thioflavin T emission curves show increased fluorescence (correlated to tau aggregation) over time when truncated tau fragment (AA297-391) (dGAE) monomer is combined with truncated tau fragment (AA297-391) (dGAE) preformed fibrils (Type 1).
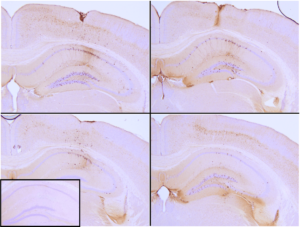
Immunohistochemical analysis of P301L mouse hippocampus injected with K18 P301L tau PFFs (SPR-330) shows seeding of tau pathology at injection site nine weeks post-injection. AT8 (pSer202/pThr205) tau antibody shows tangle-like inclusions. Inset: negative control. Experiments performed at reMYND N.V.
REFERENCES
- Tau protein modifications and interactions: their role in function and dysfunction, Mietelska-Porowska A et al, Int J Mol Sci. 2014 Mar 18;15(3):4671-713.
- Tau protein isoforms, phosphorylation and role in neurodegenerative disorders, Buée L et al, Brain Res Brain Res Rev. 2000 Aug;33(1):95-130.
- Neuropathological stageing of Alzheimer-related changes, Braak H and Braak E, Acta Neuropathol. 1991;82(4):239-59.
- Tau as a biomarker of neurodegenerative diseases, Schraen-Maschke S et al, Biomark Med. 2008 Aug;2(4):363-84.
- Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers, Lee JC et al, Exp Mol Med. 2019 May 9;51(5):1-10.
- Transcriptional Analysis of Blood Lymphocytes and Skin Fibroblasts, Keratinocytes, and Endothelial Cells as a Potential Biomarker for Alzheimer’s Disease, Mukhamedyarov MA et al, J Alzheimers Dis. 2016 Oct 18;54(4):1373-1383.


Leave a Reply