The Role of Molecular Chaperones in Neurodegenerative Diseases
Molecular chaperones have a central role in maintaining proteostasis, a term used to describe the homeostasis of the proteome. As well as assisting in processes such as polypeptide synthesis, folding, and oligomerization to ensure nascent proteins successfully reach their native states, molecular chaperones serve to direct short-lived or damaged proteins to degradation pathways.
A growing body of evidence implicates molecular chaperones in the pathogenesis of neurodegenerative diseases involving protein misfolding and aggregation. These include Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS), which are respectively characterized by intraneuronal aggregates of tau and β-amyloid, α-synuclein, and RNA-binding proteins with prion-like domains. Here, we explore some of the molecular chaperones being investigated for therapeutic targeting.
The Hsp70 family
The Hsp70 family of heat shock proteins provide a potent buffering system against cellular stresses such as nutrient deprivation and temperature fluctuation. Specifically, by transiently associating with misfolded or denatured proteins and keeping them in a folding-competent state until conditions are suitable for their release, Hsp70s help to prevent unwanted protein aggregation.
The Hsp70 family has been extensively studied for its potential utility in treating neurodegenerative diseases. In recent years, Hsp70 upregulation has been shown to enhance degradation of misfolded α-synuclein, limit the formation of α-synuclein oligomers, and decrease α-synuclein-induced cytotoxicity1. In addition, using a transient heat shock to increase Hsp70 levels in vivo has reduced β-amyloid toxicity by diminishing oligomerization2.
The Hsp110 family
The Hsp110s are a subfamily of the Hsp70 molecular chaperones, originally thought to function as nucleotide exchange factors and regulate Hsp70 activity. However, they are now known to be molecular chaperones themselves and have proven capable of binding misfolded proteins and collaborating with Hsp40 to hydrolyze ATP and return these to their native state3.
Recently, researchers from McGovern Medical School at UTHealth published a paper in the Journal of Biological Chemistry, showing a potent holdase capacity in the carboxy terminal region of Hsp110, which was predicted to contain an intrinsically disordered region (IDR). Using StressMarq’s Human Alpha Synuclein Monomers (catalog# SPR-321) and Human Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322) to perform a series of thioflavin T binding assays, they showed the IDR to inhibit the formation of both amyloid Aβ-42 and α-synuclein fibrils in vitro, indicating that it might be exploited to prevent protein aggregation and amyloidogenesis4.
Chaperonins
Chaperonins are a class of molecular chaperones that are divided into two subgroups, Groups I and II, of which the Group II chaperonins are more widely implicated in neurodegenerative diseases. The cytosolic TCP-1 Ring Complex (TRiC) is one of the best characterized Group II chaperonins, serving mainly to promote the folding of newly synthesized polypeptides presented by Hsp70 and/or prefoldin (a cochaperone).
TRiC substrates are often large, hydrophobic proteins with aggregative tendencies, and include polyQ-expanded huntingtin (the mutant protein linked to Huntingtin’s disease) as well as several other amyloidogenic proteins5. Manipulating TRiC activity is seen as a promising strategy for treating neurodegenerative diseases associated with amyloid-like inclusions.
The neuronal secretory chaperones 7B2 and proSAAS
While all cell types express the molecular chaperones just discussed, the expression of 7B2 and proSAAS is mainly restricted to neurons, neuroendocrine cells, and endocrine cells, where they are concentrated within secretory granules. Both proteins have anti-aggregant activities; for example, 7B2 has been shown to block aggregation of prohormone convertase 2 (proPC2), an enzyme which mediates the proteolytic conversion of various neuropeptide hormone precursors, while proSAAS is known to target proteins including β-amyloid, α-synuclein, and islet amyloid polypeptide.
Importantly, 7B2 and proSAAS co-localize with many of the abnormal protein deposits found in neurodegenerative diseases, including amyloid plaques and Lewy bodies, and are cytoprotective in rodent models of AD and PD. This suggests that their over expression might be used to slow disease progression6.
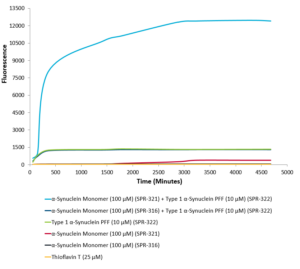
Active Human Recombinant Alpha Synuclein Pre-formed Fibrils (SPR-322) seed the formation of new alpha synuclein fibrils from a pool of Active Human Recombinant Alpha Synuclein Protein Monomers (SPR-321), as shown by a Thioflavin T assay. For full experimental details, see the product page for SPR-321.
Supporting neurodegenerative disease research
In addition to fibrilized proteins for neurodegenerative disease research, StressMarq offers a broad range of antibodies, proteins, kits, and small molecules for Hsp70, Hsp40, and other chaperones. StressMarq offers additional related products, including Hsp110 antibody (catalog# SPC-195) and TCP1-alpha Antibody (catalog# SMC-479).
Article references
[1] Chaperones in Neurodegeneration, Lindberg I et al. J Neurosci. 2015;35(41):13853-13859.
[2] Heat shock treatment reduces beta amyloid toxicity in vivo by diminishing oligomers, Wu Y et al. Neurobiol Aging. 2010;31(6):1055-1058.
[3] Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates, Mattoo RUH et al. J Biol Chem. 2013;288(29):21399-21411.
[4] Suppression of aggregate and amyloid formation by a novel intrinsically disordered region in metazoan Hsp110 chaperones, Yakubu UM and Morano KA, J Biol Chem. 2021;296:100567
[5] The role of molecular chaperones in human misfolding diseases, Broadley SA and Hartl FU, FEBS Lett. 2009;583(16):2647-2653.
[6] Secreted Chaperones in Neurodegeneration, Chaplot K et al. Front Aging Neurosci. 2020;12:268.

Leave a Reply